295,00 € – 895,00 €
Product details
Synonyms = 95kDa melanocyte-specific secreted glycoprotein, M-beta, Melanocyte lineage specific antigen GP100, Melanocyte protein Pmel 17, Melanoma associated ME20 antigen, Melanosomal matrix protein17, p100, p26, PMEL17, Premelanosome protein, Secreted melanoma-associated ME20 antigen, SILV, Silver homolog
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-617R
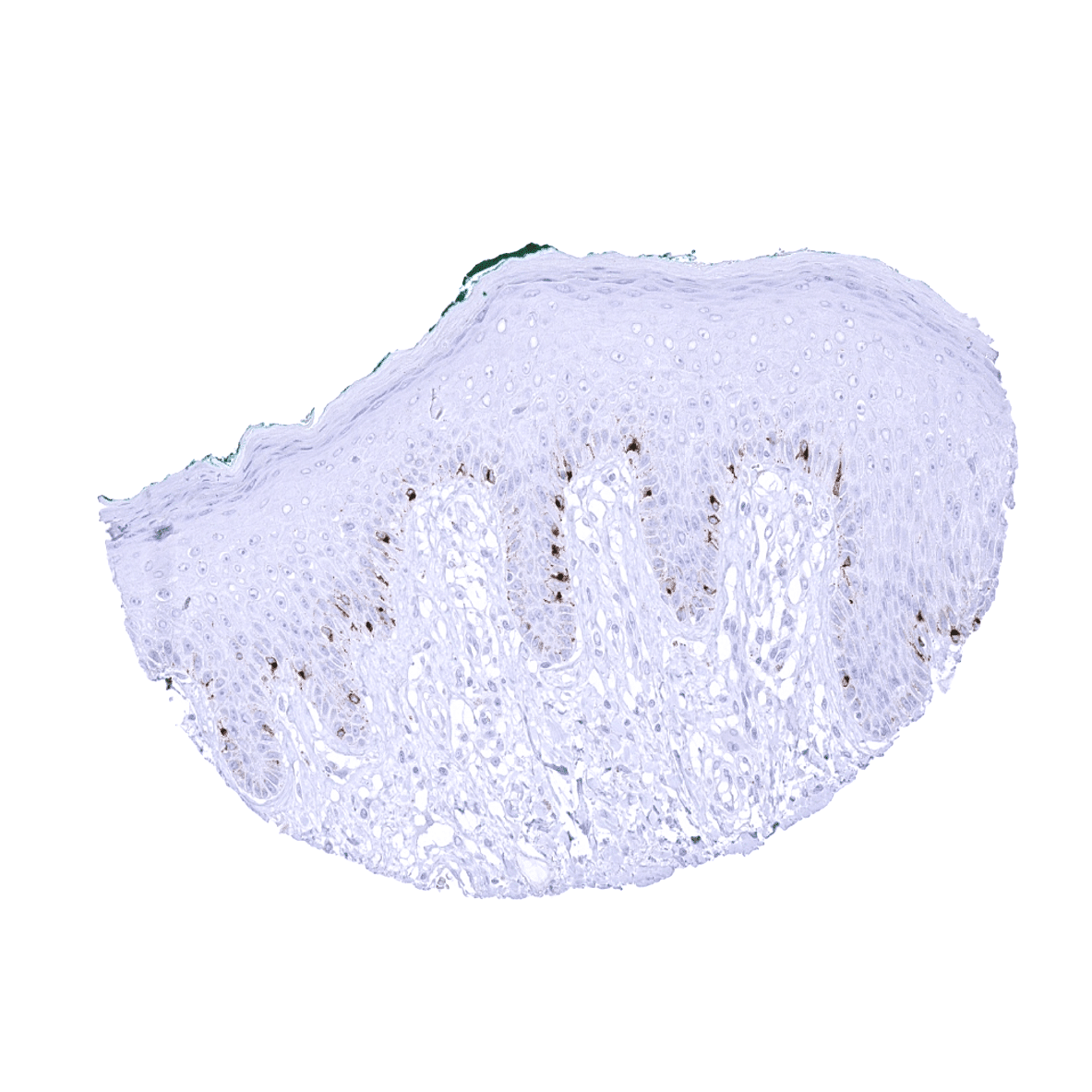
Positive control = Skin: A moderate to strong cytoplasmic PMEL immunostaining should be seen in all melanocytes of the skin.
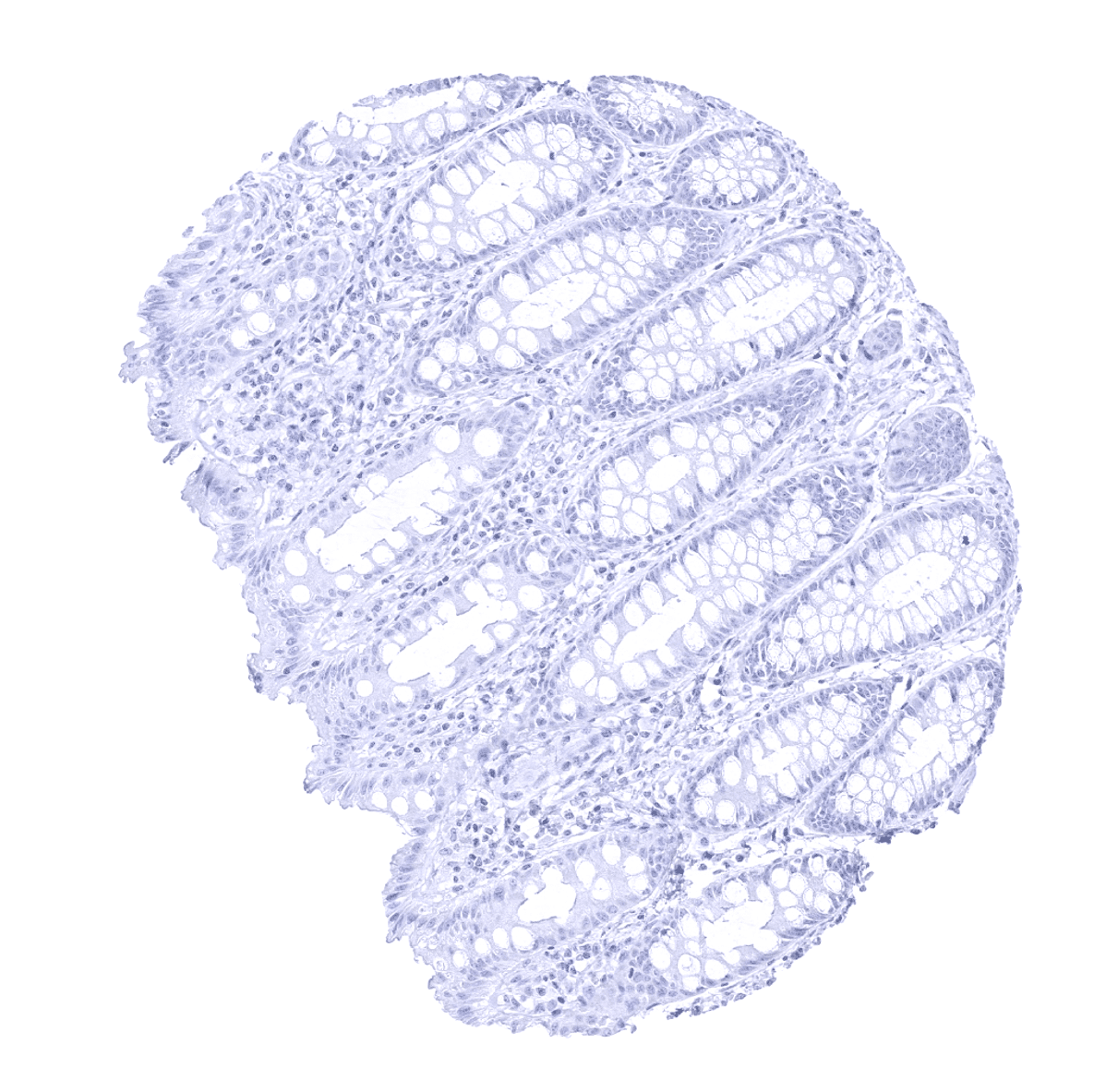
Negative control = Colon: PMEL immunostaining should be completely absent in all cells.
Cellular localization = Cytoplasmic
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:50 – 1:100
Intended Use = Research Use Only
Relevance of Antibody
PMEL 17 is expressed in normal and neoplastic melanocytes.
Biology Behind
Premelanosome protein (PMEL), also termed gp100 is a 100 kDa glycoprotein that is encoded by the PMEL gene at 12q13.2. PMEL is a pigment cell-specific protein responsible for the formation of fibrillar sheets within the pigment organelle, termed melanosome. The fibrillar sheets function as a template upon which melanins polymerize as they are synthesized. The PMEL fibrils are needed for optimal pigment cell function, as animals that either lack PMEL protein or express deficient (mutant) PMEL variants show varying extents of hypopigmentation and pigment cell impairment. The expression of the PMEL gene is regulated by the microphthalmia-associated transcription factor (MITF). The PMEL protein is a therapeutic target used in melanoma patients for vaccination and other treatment modalities.
Staining Pattern in Normal Tissues
Among normal adult tissues a cytoplasmic PMEL immunostaining is seen in melanocytes of the skin. An additional (faint) granular cytoplasmic PMEL immunostaining located immediately below the apical cell membrane can be seen in gallbladder epithelium. This is considered a tolerable antibody cross-reactivity.
These findings are largely consistent with the RNA and protein data described in the Human Protein Atlas (Tissue expression PMEL)
Positive control: Skin: A moderate to strong cytoplasmic PMEL immunostaining should be seen in all melanocytes of the skin.
Negative control: Colon: PMEL immunostaining should be completely absent in all cells.
Staining Pattern in Relevant Tumor Types
A positive PMEL immunostaining is seen in the majority of malignant melanomas. In benign melanocytic naevus, the epidermal part may be strongly stained while the dermal part is weakly stained or negative. PMEL is also seen in most cases of blue naevus, cellular blue naevus, dysplastic naevus, and Spitz naevus. PMEL also occurs in various other neoplasms of melanocytic origin or differentiation, such as for example PEComas derived from modified smooth muscle cells in the tuberous sclerosis complex (angiomyolipoma, lymphangioleiomyoma(-tosis), pulmonary sugar tumor, cardiac rhabdomyoma).
The TCGA findings on PMEL RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply MSVA-617R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-617R 1:150 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-617R 1:150 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Roche – Ventana Discovery ULTRA
Pretreatment for 64 minutes at 100°C (pH 8,4); CM peroxidase blocking for 12 minutes (room temperature), MSVA-617R 1:150 for 20 minutes at 36°C, secondary antibody (anti-rabbit HQ) for 12 minutes at 36°C, anti-HQ HRP for 12 minutes at room temperature, DAB at room temperature, hematoxylin II at room temperature for 8 minutes, bluing reagent at room temperature for 4 minutes.
These images depict staining results obtained by the protocol described above. It is of note, that the Ventana machines generally require higher antibody concentrations than other commonly used autostainers because the antibodies are automatically diluted during the procedure. Various other protocols can result in an identical result as shown above. A longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of secondary antibody and or the anti-HQ HRP result in stronger staining, potentially at the cost of more background staining.
Potential Research Applications
- PMEL is a promising therapeutic target used by various treatment modalities. Its therapeutic potential is still under investigation.
- PMEL expression is not detected in all malignant melanomas and the expression levels vary between individual tumors. The prognostic/clinical impact of PMEL expression levels is unclear in melanomas and should be investigated.
Evidence for Antibody Specificity in IHC
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
Orthogonal validation: For the antibody MSVA-617R, specificity is suggested by the strong concordance of its immunostaining data with data from three independent RNA screening studies, including the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project, which are all summarized in the Human Protein Atlas (Tissue expression PMEL). Immunostaining by using MSVA-617R was almost exclusively detected in the skin, for which PMEL RNA expression had been documented. Low level RNA expression described for the uterine cervix and endometrium could not be detected in an immunohistochemical analysis using MSVA-617R. However, cervix and endometrium PMEL immunostaining were also not observed by using the antibody HPA031649 . An additional faint PMEL immunostaining can sometimes be seen for MSVA-617R in gallbladder epithelium. This staining is cytoplasmic, granular and located immediately below the apical cell membrane. It is considered a tolerable antibody cross-reactivity.
Comparison of antibodies: Specificity of MSVA-617R immunostaining of melanocytes of the skin is further suggested by an identical staining seen by using HPA031649 . Our notion that MSVA-617R staining of gallbladder epithelium reflects a cross-reactivity is corroborated by a comparison with the PMEL staining obtained by the antibody HPA031649. These HPA031649 incubations did not result in a comparable gallbladder staining. Given the characteristic granular pattern of the gallbladder cross-reactivity and the rarity of melanomas in the gallbladder, we consider this cross-reactivity tolerable.