Product details
Synonyms = bHLHc1, Class C basic helix-loop-helix protein 1, Myoblast determination protein 1, Myogenic differentiation 1, Myogenic factor 3 (Myf-3), Myogenin D1, PUM
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-801R
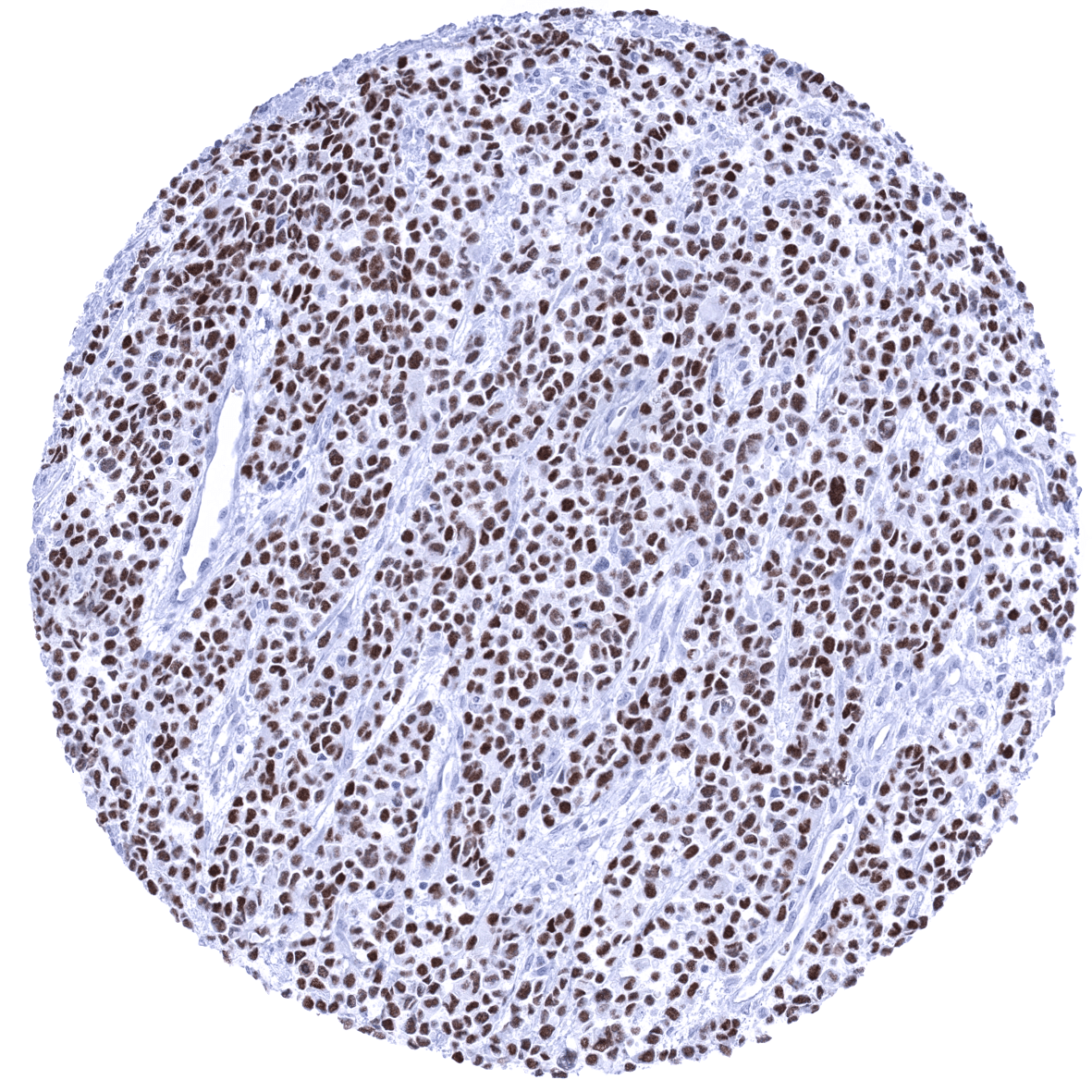
Positive control = Rhabdomyosarcoma with previously documented MyoD1 expression should demonstrate a moderate to strong nuclear MyoD1 immunostaining.
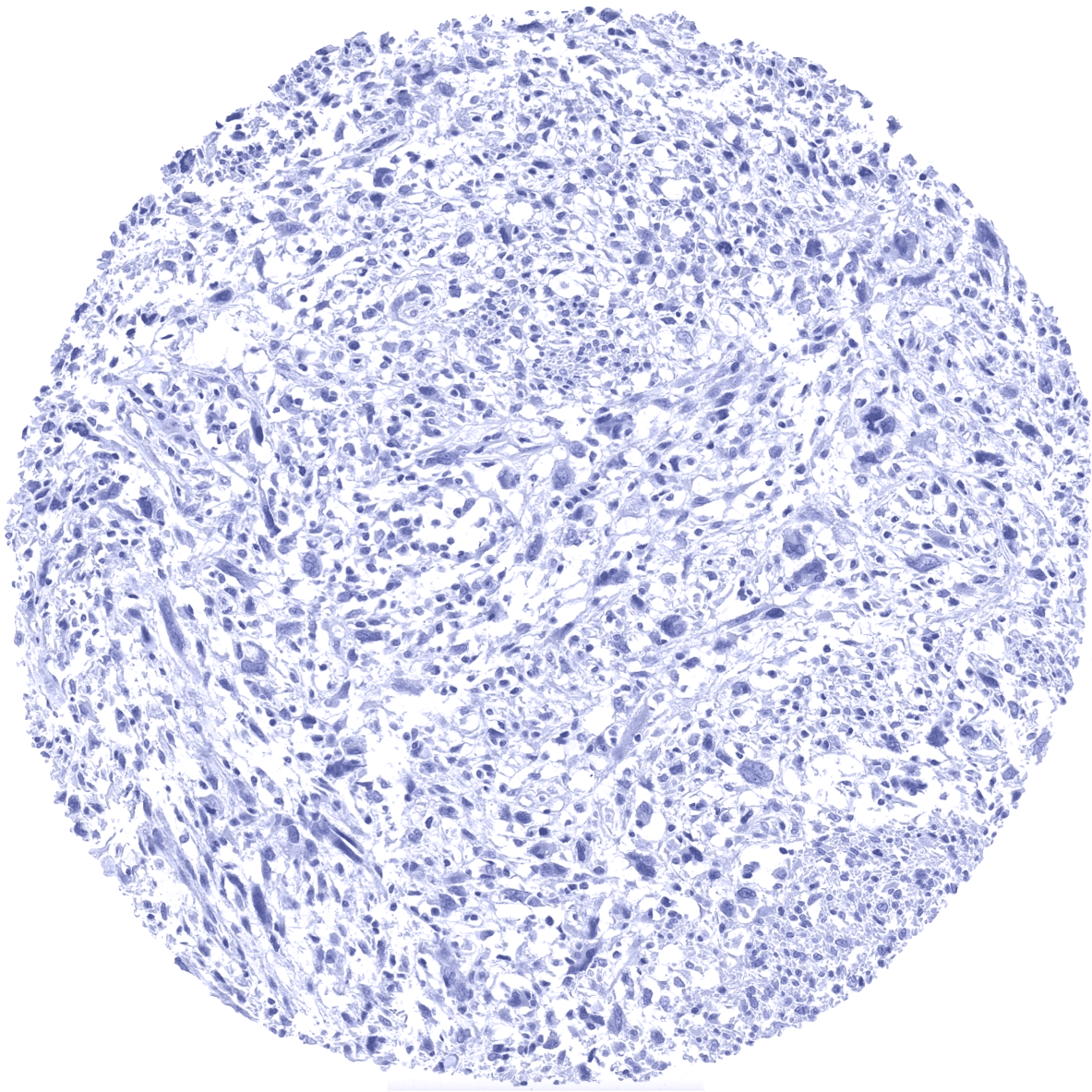
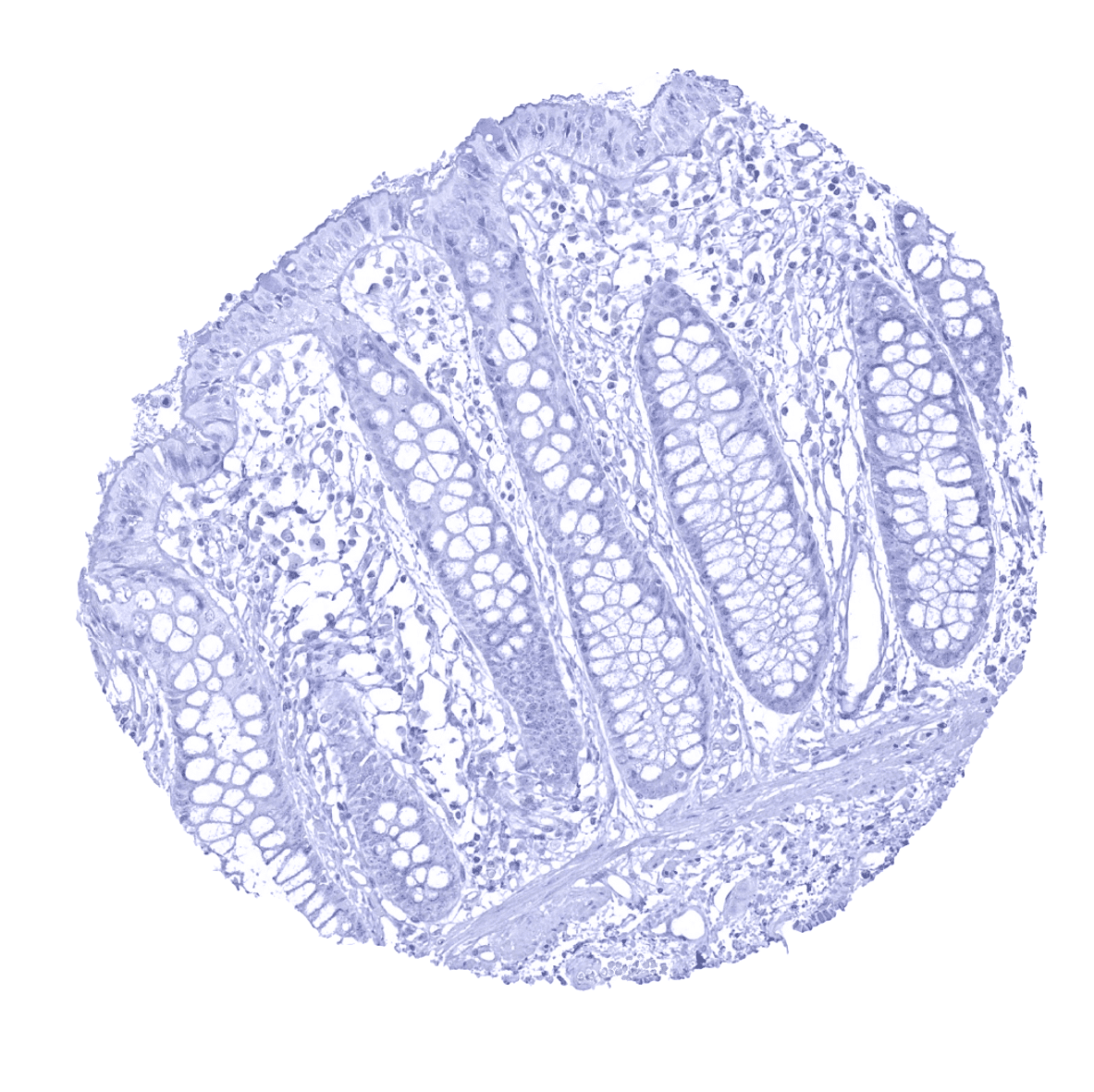
Negative control = Colon or tonsil: MyoD1 immunostaining (nuclear, membranous, and cytoplasmic) should be completely absent in all cell types.
Cellular localization = Nuclear
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
MyoD1 is expressed in the early phase of myogenic differentiation.
Biology Behind
Myoblast determination protein 1 (MyoD1) is coded by a gene on 11p15.1. MyoD1 belongs to a protein family of myogenic regulatory factors (MRFs) also including Myf5, myogenin, and MRF4 (Myf6). MyoD1 is one of the earliest markers of myogenic commitment. MyoD1 plays an important role in the regulation of muscle differentiation. Its expression commits mesoderm cells to a skeletal myoblast lineage. MyoD1 is normally expressed in muscle stem cells (myosatellite cells) at extremely low (undetectable) levels, but expression of MyoD1 is activated in response to exercise or muscle tissue damage. MyoD1 cooperates with the tumor suppressor gene, Retinoblastoma (pRb) to induce cell cycle arrest in terminally differentiated myoblasts. Due to functional redundancy with Myf5 and/or Mrf4, muscle development is not markedly ablated in mouse mutants lacking the MyoD1 gene.
Staining Pattern in Normal Tissues
Among normal adult tissues nuclear MyoD1 immunostaining is not observed. Nuclear staining can only be seen in normal fetal muscle. Using MSV-801R, a granular cytoplasmic attaining of variable intensity occurs in pancreatic acinar cells in a fraction of samples and a weak membranous staining of the surface membrane of the syncytiotrophoblast can be found in some samples of the placenta. These stainings reflect a (tolerable) cross reactivity which compares favorably to the cross-reactivity spectrum of other MyoD1 antibodies.
These findings are largely consistent with the RNA and protein data described in the Human Protein Atlas (Tissue expression MyoD1)
Positive control: Rhabdomyosarcoma with previously documented MyoD1 expression should demonstrate a moderate to strong nuclear MyoD1 immunostaining.
Negative control: Colon or tonsil: MyoD1 immunostaining (nuclear, membranous, and cytoplasmic) should be completely absent in all cell types.
Staining Pattern in Relevant Tumor Types
A positive MyoD1 immunostaining is seen in most cases of rhabdomyosarcoma. Rarely, a positive MyoD1 staining can also occur in various other tumor entities such as for example mesenchymal chondrosarcomas or sarcomatoid carcinomas.
The TCGA findings on MyoD1 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
-Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply MSVA-801R at a dilution of 1:100 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Potential Research Applications
- The clinical significance of MyoD1 expression levels in rhabdomyosarcomas needs to be investigated.
- The prevalence (and possible clinical significance) of aberrant MyoD1 staining in non-myogenic tumors has not been systematically evaluated.
Evidence for Antibody Specificity in IHC
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
Orthogonal validation: For the antibody MSVA-801R orthogonal validation by comparing the normal tissue immunostaining data with data from three independent RNA screening studies, including the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project, which are all summarized in the Human Protein Atlas (Tissue expression MyoD1) does result in concordant results. Immunostaining by using MSVA-801R is not seen in skeletal muscle (and tongue) as suggested by RNA expression data. This is not surprising, because of the low level of RNA expression which is unlikely to result in detectable protein expression. True detection of MyoD1 by MSVA-801R is suggested by the strong nuclear positivity seen in 3 of 4 analyzed rhabdomyosarcomas (images of all of these cases are shown in the tumor gallery). Non-nuclear MyoD1 immunostaining is sometimes seen for MSVA-801R in pancreatic acinar cells (granular cytoplasmic) and in mature placenta (membranous, superficial membrane of syncytiotrophoblast). These findings are considered a (tolerable) cross-reactivity.
Comparison of antibodies: That the cytoplasmic and membranous staining of pancreas and placenta by MSVA-801R reflects a cross-reactivity was confirmed by a comparison with another commercial anti-MyoD1 antibody RTU (IVD) showing a different cross-reactivity staining pattern in pancreatic cells and lacking membranous staining in the placenta (see antibody comparison below). The staining for the RTU (IVD) antibody was done by using an autostainer and the recommended protocol.
Antibody Comparison: MSVA-801R vs another commercial anti-MyoD1 antibody RTU (IVD)