195,00 € – 695,00 €
Product details
Synonyms = Cell Proliferation-inducing Gene 46 Protein; CK18; CYK18Cytokeratin Endo B; K18; Keratin-18; Kerd; KRT18
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-118R
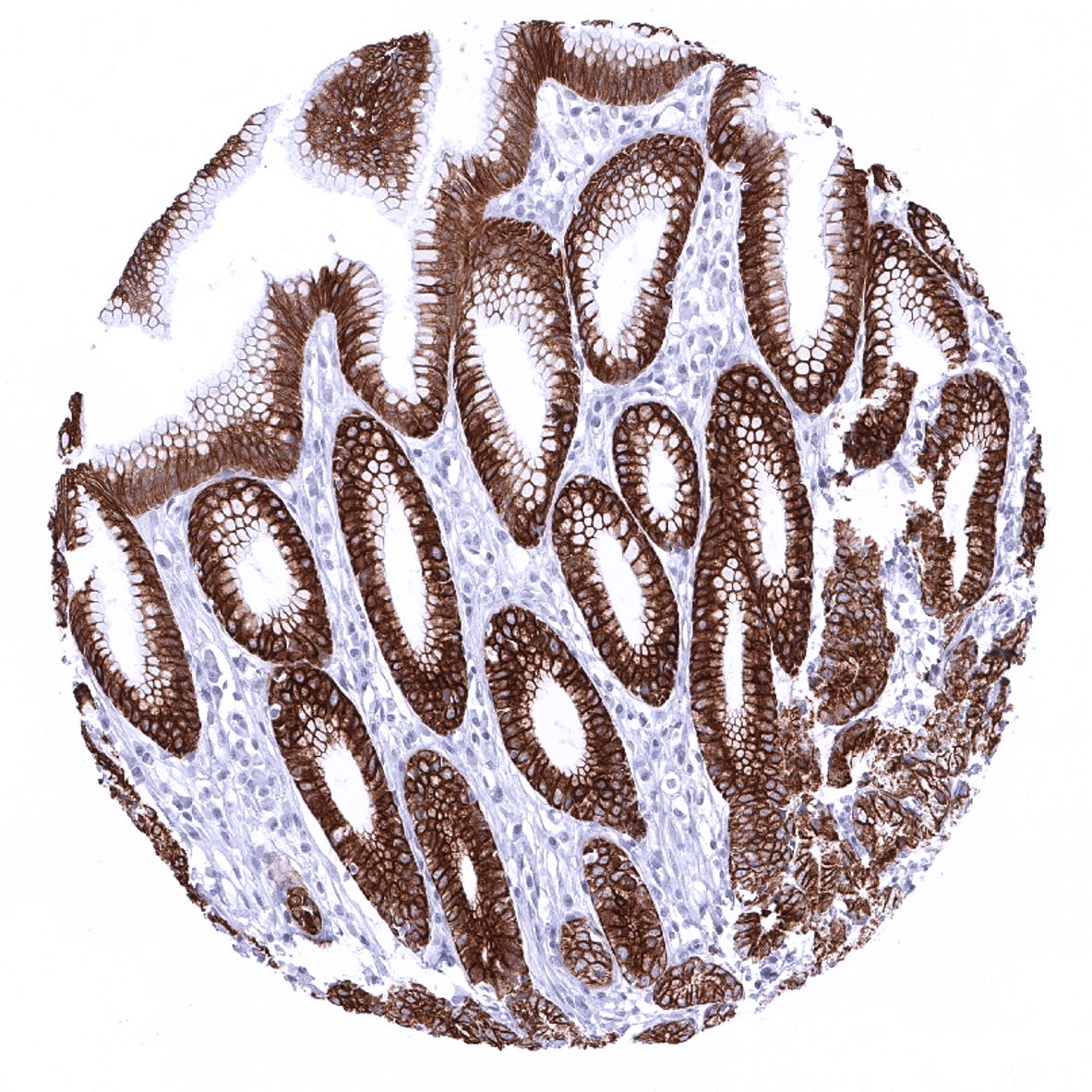
Positive control = Liver: At least a weak to moderate, predominantly membranous staining should be seen in virtually all hepatocytes. Appendix: A strong staining should be seen in all epithelial cells.
Negative control = Appendix: Staining should be absent in all non-epithelial cells of the appendix.
Cellular localization = Cytoplasmic
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
Biology Behind
Cytokeratin 18 (CK18), also termed keratin 18 (KRT18) is an acidic type I keratin protein encoded by the KRT18 gene on 12q13. It forms heteropolymers with his co-expressed complementary type II keratin partner KRT8, which – assembled into keratin filaments – shape the cytoskeleton of many epithelial cell types. As other cytokeratins, KRT18 is part of the cytoskeletal scaffold within epithelial cells, which contributes to the cell architecture and provides the cells with the ability to withstand mechanical stress. CK18 is primarily expressed in single-layered or “simple” epithelial tissues. Beside the important structural function, CK18 was also suggested to play a role in the regulation of cancer associated processes such as apoptosis, cell cycle progression, and cancer-related signaling pathways.[1]
[1] Menz et al. “Diagnostic and prognostic impact of cytokeratin 18 expression in human tumors. a tissue microarray study on 11,952 tumors” Molecular Medicine 27, Article number: 16 (2021)
Staining Pattern in Normal Tissues
Cytokeratin 18 staining pattern in Normal Tissues with antibody MSVA-118R (images are shown in our “Normal Tissue Gallery”)
| Brain | Cerebrum | Negative. |
| Cerebellum | Negative. | |
| Endocrine Tissues | Thyroid | Moderate to strong staining of follicular cells. |
| Parathyroid | Moderate staining of all epithelial cells. | |
| Adrenal gland | Negative. | |
| Pituitary gland | Strong staining of epithelial cells in the adenohypophysis. | |
| Respiratory system | Respiratory epithelium | Strong CK18 positivity of epithelial cells but staining may be weaker in basal cells. |
| Lung | Strong CK18 positivity of all pneumocytes. | |
| Gastrointestinal Tract | Salivary glands | CK18 staining is strong in serous and mucinous cells but is generally weaker in excretory ducts. Individual excretory ducts also show weak or absent staining. |
| Esophagus | Negative. | |
| Stomach | Strong CK18 positivity of most epithelial cells. Staining is weak or absent in parietal cells of the corpus. | |
| Duodenum | Strong CK18 positivity of all epithelial cells. | |
| Small intestine | Strong CK18 positivity of all epithelial cells. | |
| Appendix | Strong CK18 positivity of all epithelial cells. | |
| Colon | Strong CK18 positivity of all epithelial cells. | |
| Rectum | Strong CK18 positivity of all epithelial cells. | |
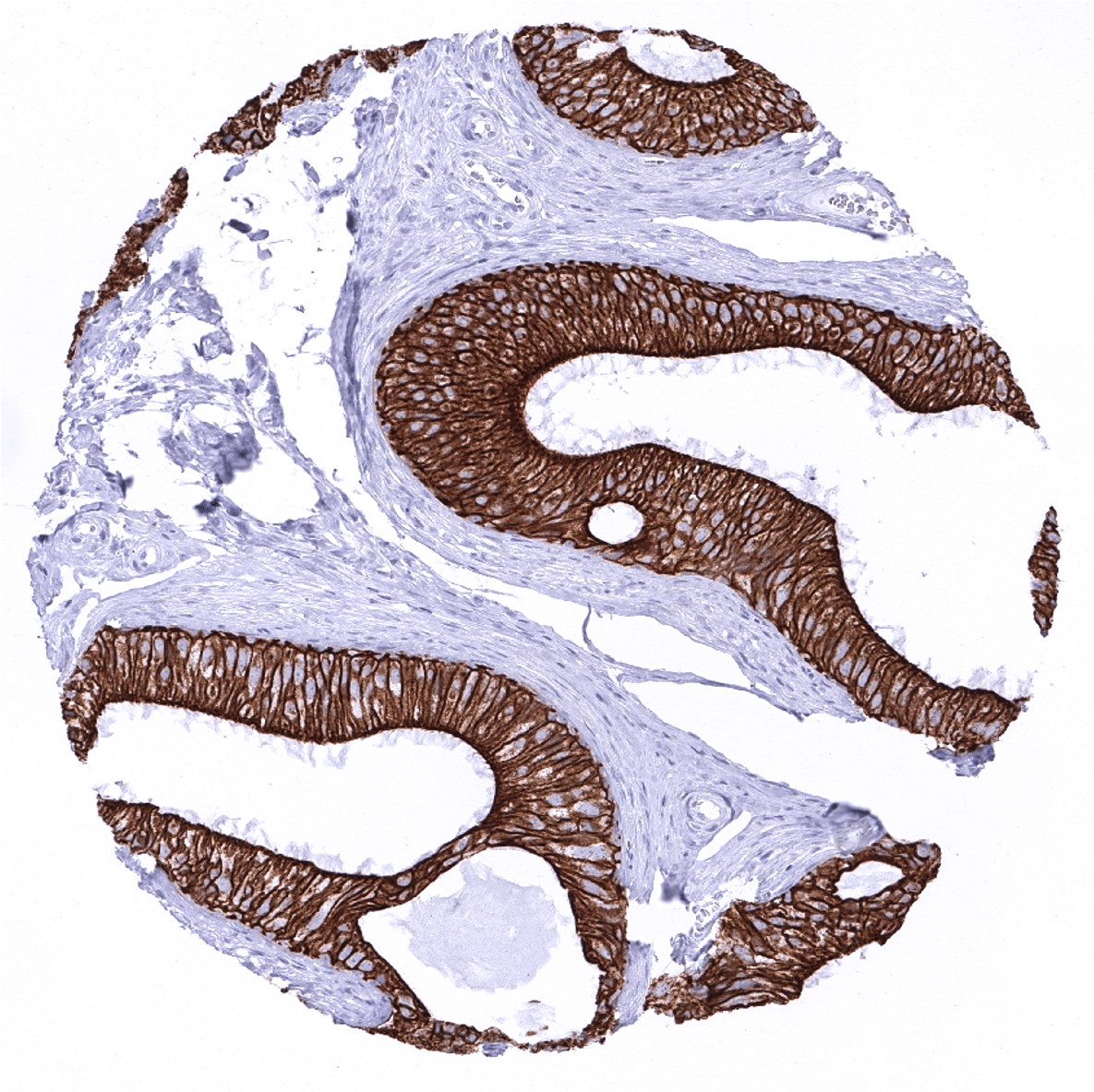
| Liver | Strong CK18 positivity of bile ducts. Staining shows a zonal variability in hepatocytes ranging from negative to moderately positive. | |
| Gallbladder | Strong CK18 positivity of all epithelial cells. | |
| Pancreas | Strong CK18 positivity of all epithelial cells but weaker in islet cells. | |
| Genitourinary | Kidney | Strong CK18 positivity of proximal tubuli, distal tubuli and collecting ducts. |
| Urothelium | Umbrella cells exhibit a strong CK18 staining. Staining intensity decreases gradually from superficial to basal cells. Basal cells can be CK18 negative. | |
| Male genital | Prostate | Strong CK18 positivity of all glandular cells. Staining may be weaker in basal cells. |
| Seminal vesicles | Strong CK18 positivity of all epithelial cells. Staining may be weaker in basal cells. | |
| Testis | Negative. | |
| Epididymis | Strong CK18 positivity of all epithelial cells. Staining may be weaker in basal cells. | |
| Female genital | Breast | Strong CK18 positivity of all epithelial cells. Staining may be weaker in myoepithelial cells. |
| Uterus, myometrium | Negative. | |
| Uterus, ectocervix | Negative. | |
| Uterus endocervix | Strong CK18 positivity of all epithelial cells. | |
| Uterus, endometrium | Strong CK18 positivity of all epithelial cells. | |
| Fallopian Tube | Strong CK18 positivity of epithelial cells. | |
| Ovary | Follicular cells and some cells of the corpus luteum are CK18 positive. | |
| Placenta early | Strong CK18 positivity of Syncytiotrophoblast and cytotrophoblast. | |
| Placenta mature | Strong CK18 positivity of Syncytiotrophoblast and cytotrophoblast. | |
| Amnion | Weak to moderate CK18 positivity. | |
| Chorion | Strong CK18 positivity of chorion cells. | |
| Skin | Epidermis | Negative. |
| Sebaceous glands | Negative. | |
| Muscle/connective tissue | Heart muscle | Negative. |
| Skeletal muscle | Negative. | |
| Smooth muscle | Negative. | |
| Vessel walls | Negative. | |
| Fat | Negative. | |
| Stroma | Negative. | |
| Endothelium | Negative. | |
| Bone marrow/ lymphoid tissue | Bone marrow | Negative. |
| Lymph node | A fibrillar staining CK18 staining – predominantly seen in the interfollicular area – is due fibroblastic reticulum cells. | |
| Spleen | Negative. | |
| Thymus | A fibrillar staining CK18 staining – predominantly seen in the interfollicular area – is due fibroblastic reticulum cells. Few cells within corpuscles of Hassall’s are CK18 positive. | |
| Tonsil | A fibrillar staining CK18 staining – predominantly seen in the interfollicular area – is due fibroblastic reticulum cells. | |
| Remarks |
These findings are largely comparable to the RNA and protein data described and summarized in the Human Protein Atlas (Tissue expression Cytokeratin 18).
Suggested positive tissue control: Liver: At least a weak to moderate, predominantly membranous staining should be seen in virtually all hepatocytes. Appendix: A strong staining should be seen in all epithelial cells.
Suggested negative tissue control: Appendix: Staining should be absent in all non-epithelial cells of the appendix.
Staining Pattern in Relevant Tumor Types
KRT18 expression is detectable in many different tumor types. High level KRT18 expression commonly occurs in many epithelial tumors, mainly in those derived from KRT18 positive normal tissues. Tumor types which are almost always KRT18 positive include for example adenocarcinomas of the colorectum, stomach, lung, prostate, pancreas, thyroid as well as ovarian cancer. Tumor types that are mostly KRT18 negative for example include squamous cell carcinomas from various sites of origin, seminomas, and soft tissue tumors. Loss of KRT18 expression in cancers derived from KRT18 expressing precursor cells and upregulation or neoexpression of KRT18 in neoplasias derived from KRT18 negative precursor cells is often linked to unfavorable tumor phenotype and poor patient outcome.
Detailed data on CK18/KRT18 staining by MSV-118R obtained from an analysis of 11,952 tumors from 115 different tumor types and subtypes have recently been published by Menz et al. “Diagnostic and prognostic impact of cytokeratin 18 expression in human tumors. a tissue microarray study on 11,952 tumors” Molecular Medicine 27, Article number: 16 (2021)
The TCGA findings on Cytokeratin 18 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
Cytokeratin 18 (MSVA-118R) publication summary (MSVA-118R is the recombinant version of the published antibody clone MSVA-118M)
Relevant publication: Menz et al. “Diagnostic and prognostic impact of cytokeratin 18 expression in human tumors. a tissue microarray study on 11,952 tumors” Molecular Medicine 27, Article number: 16 (2021)
A total of 11952 tumors were analyzed from 115 different tumor categories by using the following protocol: Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH7,8 Target Retrieval Solution buffer. MSVA-118R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent). This protocol was also used for all stainings depicted in our tumor and normal tissue galleries.
At least one case with a positive cytokeratin 18 immunostaining was seen in 90 (78,3%) and at least one case with a strong cytokeratin 18 immunostaining was seen in 78 (67,8%) of 115 tumor categories. The distribution of positive staining results is shown “organ-systematic” and in a “ranking order” figure below (images based on data from Menz et al). Results on possible associations with histopathological and clinical parameters of tumor aggressiveness in several cancer types are also summarized below (table based on data from Menz et al).
Authors conclusions on diagnostic utility with respect to the distinction of benign versus malignant (Menz et al):
- A positive CK18 immunostaining in squamous epithelium (usually CK18 negative) may herald neoplastic transformation (more studies needed).
- The close to 100% prevalence of CK18 expression in gastrointestinal cancers supports the concept of using CK18 immunostaining for metastasis detection
Authors conclusions on diagnostic utility with respect to the distinction of different tumor entities (Menz et al):
- The most useful diagnostic application of CK18 IHC may be the distinction of seminomas from other germ cell tumors of the testis. Only 12 of 204 analyzed seminomas (6%) but all of 88 embryonal carcinomas and yolk sac tumors of the testis showed CK18 expression.
Authors conclusions on prognostic/predictive role of CK18 expression (Menz et al.):
- Loss of CK18 expression in tumors derived from CK18 expressing precursor cells (i.e. clear cell kidney cancer, breast cancer) is often linked to unfavorable tumor features and/or poor prognosis.
- Upregulation/neo-expression of CK18 may be linked to unfavorable tumor features and/or poor prognosis in squamous cell carcinomas derived from various different organs.
Data from the publication: “Diagnostic and prognostic impact of cytokeratin 18 expression in human tumors. a tissue microarray study on 11,952 tumors” Published by Menz et al. Summarized in own graphics:
Figure 1. CK18 staining in cancer (“organ-systematic”; according to Menz et al.)
Figure 2. CK18 staining in cancer (“ranking list”; according to Menz et al.)
Figure 3. Clinico-pathological associations described by Menz et al. (p-value)
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply MSVA-118R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-118R 1:150 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-118R 1:150 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Roche – Ventana Discovery ULTRA
Pretreatment for 64 minutes at 100°C (pH 8,4); CM peroxidase blocking for 12 minutes (room temperature), MSVA-118R 1:150 for 20 minutes at 36°C, secondary antibody (anti-rabbit HQ) for 12 minutes at 36°C, anti-HQ HRP for 12 minutes at room temperature, DAB at room temperature, hematoxylin II at room temperature for 8 minutes, bluing reagent at room temperature for 4 minutes.
These images depict staining results obtained by the protocol described above. It is of note, that the Ventana machines generally require higher antibody concentrations than other commonly used autostainers because the antibodies are automatically diluted during the procedure. Various other protocols can result in an identical result as shown above. A longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of secondary antibody and or the anti-HQ HRP result in stronger staining, potentially at the cost of more background staining.
Impact of pH
MSVA-118R results in strongest staining if pH9,0 is used for slide pretreatment. pH7,8 is acceptable but lower pH results in a significant deterioration of sensitivity. An example of the impact of pH is shown below for normal kidney tissue. Despite a slightly stronger staining after slide pretreatment at pH9 as compared to pH7.8, users applying manual protocols may prefer pH7,8 because tissue damage is reduced.
Potential Research Applications
- The diagnostic utility of KRT18 expression analysis should be investigated in a large cohort of tumors from different entities.
- Loss of CK18 expression in tumors derived from CK18 positive progenitor cells and neo-expression of CK18 in tumors derived from CK18 negative progenitor cells have been linked to poor prognosis. The extent of clinical utility of these observations needs further research.
Evidence for Antibody Specificity in IHC
Specificity of MSVA-118R is documented by strong positive staining in cell types that are well documented to express KRT18 such as glandular epithelium of various origins and absence of staining in all tissues known to not express KRT18 such as squamous epithelia as well as hematopoietic cells and soft tissues.