295,00 € – 995,00 €
Product details
Synonyms = VIM
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-458R
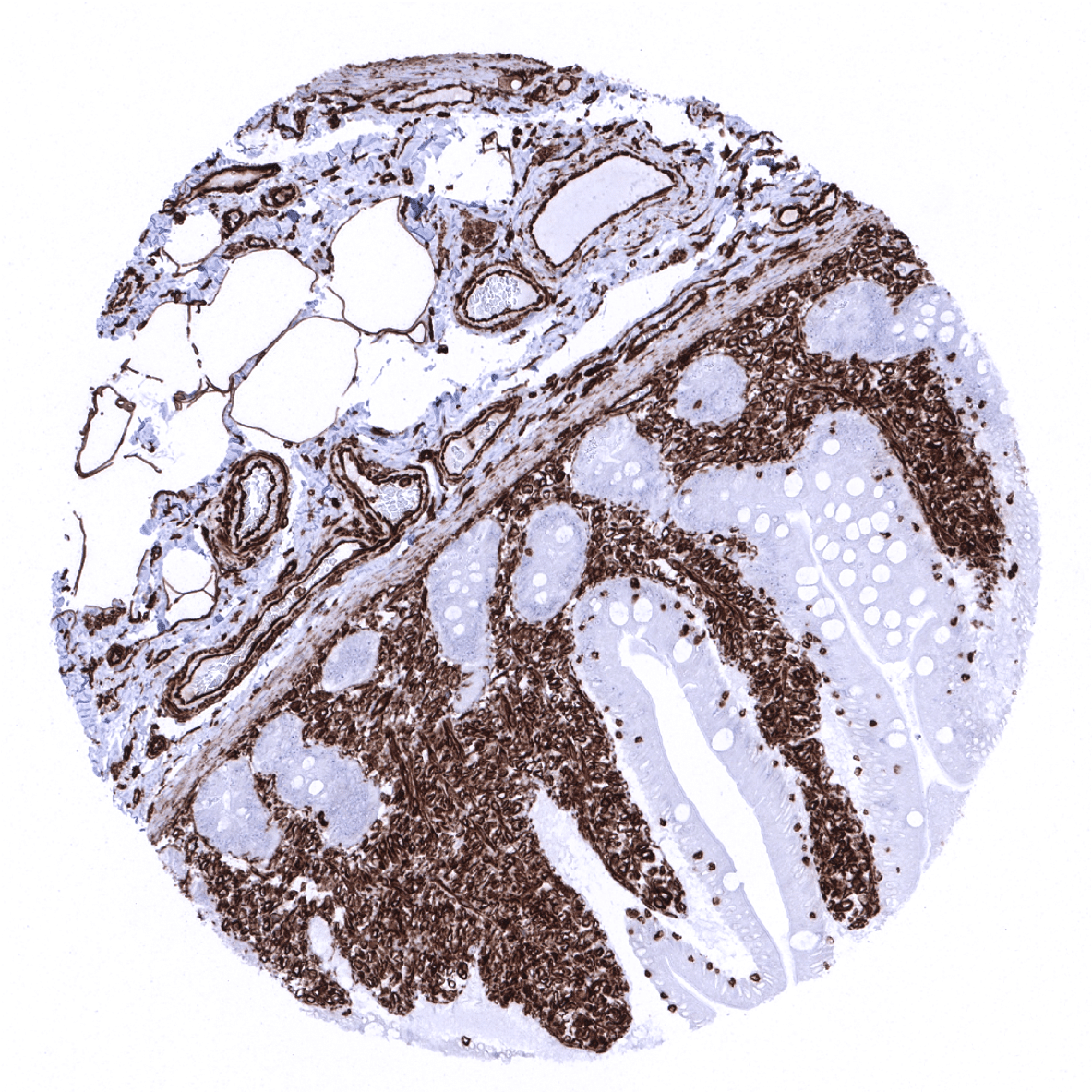
Positive control = Liver: Kupffer cells must show a strong staining and sinusoidal endothelial cells should show an at least a weak staining. Colon: Endothelial and muscle cells of large vessels and stromal cells must show a strong Vimentin staining while dispersed intraepithelial T-cells must show an at least moderate intensity staining.
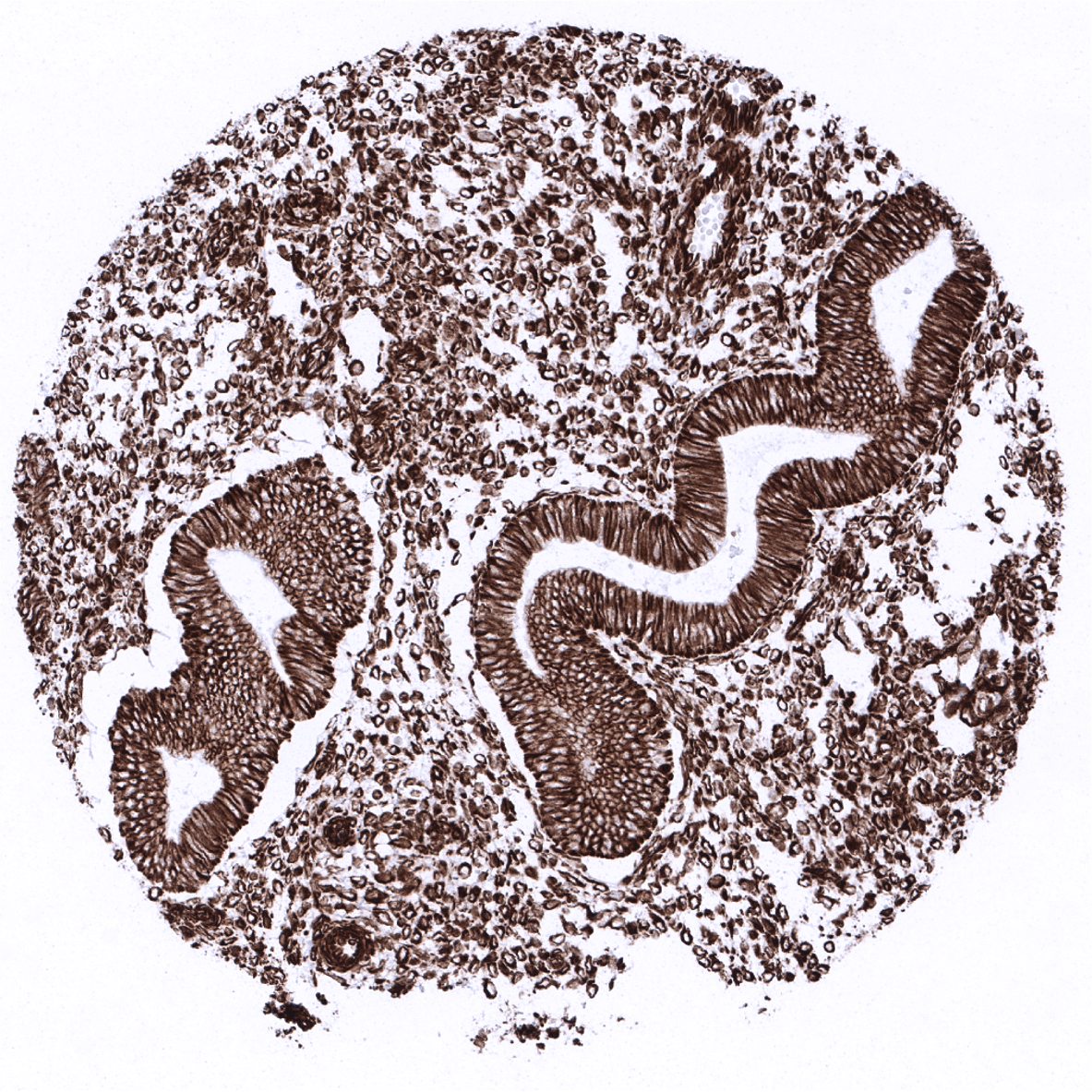
Negative control = Liver: Hepatocytes cells must be Vimentin negative. Colon: All epithelial cells must be negative.
Cellular localization = Cytoplasmic
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
Biology Behind
Vimentin is a 57 kDa protein coded by the VIM gene at 10p13. It is the first intermediate filament protein to be expressed during cell differentiation. All primitive cell types express vimentin but in most non-mesenchymal cells it is later replaced by other intermediate filament proteins. In non-vascular smooth muscle cells and in striated muscle, vimentin is often replaced by desmin, but vimentin remains the major intermediate filament seen in non-muscle cells. It can be re-expressed in muscle cells in case of injury and regeneration. Vimentin is required for stabilizing the position of the organelles in the cytosol. Vimentin provides cells with resilience and is considered the cytoskeletal component responsible for maintaining cell integrity. Transgenic mice that lack vimentin are viable although they show various developmental defects and delayed wound healing. Vimentin is the major cytoskeletal component of mesenchymal cells and is thus used as a marker of mesenchymal cell origin and of epithelial-to-mesenchymal transition (EMT) during cancer progression. Vimentin expression induces a transformation of cells to an elongated, flat, mesenchymal shape.
Staining Pattern in Normal Tissues
Vimentin immunostaining is found in various mesenchymal cells including fat cells, fibroblasts, endothelial cells, macrophages, melanocytes, Langerhans cells, Schwann cells, glial cells, lymphocytes, mesothelium, ovarian granulosa cells, Sertoli and Leydig cells of the testis. Vimentin is usually absent in skeletal and heart muscle, but regularly seen in vascular smooth muscle. In non-vascular smooth muscle vimentin expression is normally low or absent, but can be upregulated in case of regeneration. Vimentin is also regularly found in several specialized epithelia, such as the Bowman capsule of the kidney, fallopian tube, endometrium, endocervix (weak), thyroid gland, adrenal gland (cortex and medulla), and pancreas (basolateral portion of acinar cells) as well as in myoepithelial cells of the breast, salivary and sweat glands. Vimentin can occasionally also be seen in some normal appearing prostate acinar cells, principal cells of the epididymis, scattered respiratory epithelial cells, glandular cells of Brunner glands of the duodenum. Vimentin immunostaining is largely absent in epithelial cells of the gastrointestinal tract, luminal breast epithelial cells, mucinous and serous cells in salivary glands, parathyroid gland, pneumocytes, trophoblastic cells of early and mature placenta, hepatocytes, proximal and distal tubules as well as collecting ducts of the kidney, cells of the spermatogenesis including mature sperms, neurons and nerve fibres. Vimentin is absent in cells of the adenohypophysis but strongly expressed in the neurohyophysis.
These findings are largely comparable to the RNA and protein data described in the Human Protein Atlas (Tissue expression Vimentin).
Suggested positive tissue control: Liver: Kupffer cells must show a strong staining and sinusoidal endothelial cells should show an at least a weak staining. Colon: Endothelial and muscle cells of large vessels and stromal cells must show a strong Vimentin staining while dispersed intraepithelial T-cells must show an at least moderate intensity staining.
Suggested negative tissue control: Liver: Hepatocytes cells must be Vimentin negative. Colon: All epithelial cells must be negative.
Staining Pattern in Relevant Tumor Types
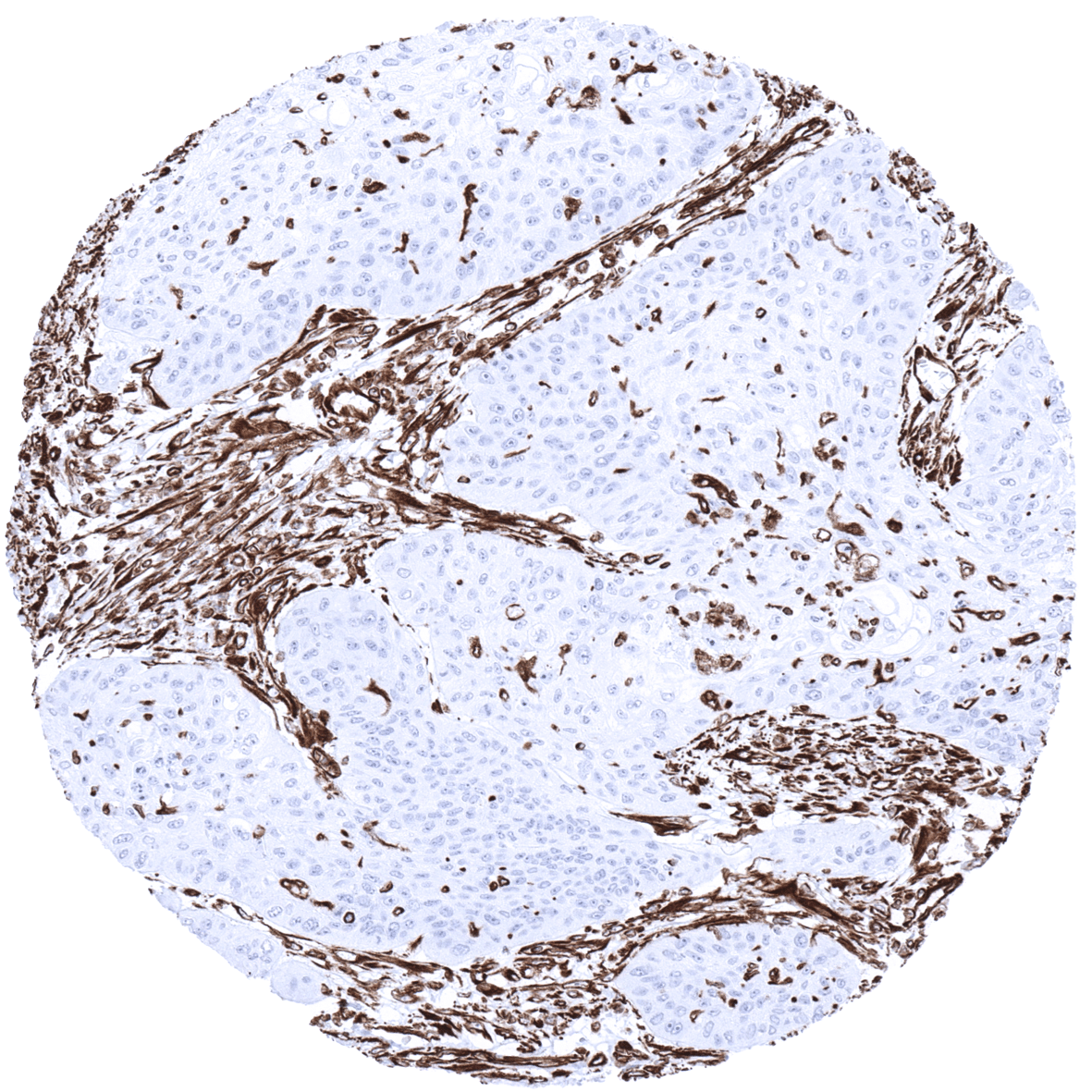
Vimentin is present in many different neoplasms but is particularly expressed in those originated from mesenchymal cells. Sarcomas e.g., fibrosarcoma, angiosarcoma, and leio- and rhabdomyosarcoma, sarcoma NOS, as well as lymphomas, malignant melanoma and schwannoma, are virtually always vimentin positive. Mesoderm derived carcinomas like renal cell carcinoma, adrenal cortical carcinoma and adenocarcinomas from endometrium and ovary usually express vimentin. Also thyroid carcinomas are vimentin positive. Any poorly differentiated or sarcomatoid carcinoma may express some vimentin.
The TCGA findings on Vimentin RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein. Accordingly, multiple different protocols can generate identical staining results.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH9 Target Retrieval Solution buffer. Apply MSVA-458R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-458R 1:150 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-458R 1:150 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Roche – Ventana Discovery ULTRA
Pretreatment for 64 minutes at 100°C (pH 8,4); CM peroxidase blocking for 12 minutes (room temperature), MSVA-458R 1:75 for 20 minutes at 36°C, secondary antibody (anti-rabbit HQ) for 12 minutes at 36°C, anti-HQ HRP for 12 minutes at room temperature, DAB at room temperature, hematoxylin II at room temperature for 8 minutes, bluing reagent at room temperature for 4 minutes.
These images depict staining results obtained by the protocol described above. It is of note, that the Ventana machines generally require higher antibody concentrations than other commonly used autostainers because the antibodies are automatically diluted during the procedure. Various other protocols can result in an identical result as shown above. A longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of secondary antibody and or the anti-HQ HRP result in stronger staining, potentially at the cost of more background staining.
Potential Research Applications
- Vimentin is a canonical marker for epithelial-mesenchymal transformation. As such it can be used in multicolor imaging for delineating and studying cells undergoing EMT.
Evidence for Antibody Specificity in IHC
Specificity of MSVA-458R is documented by strong positive staining in all mesenchymal cell types that are well documented to express vimentin and absence of staining in skeletal and heart muscle as well as in these epithelial tissues known to not express vimentin such as for example colon epithelium and hepatocytes.