295,00 € – 895,00 €
Product details
Synonyms = AITD3, hTG, TDH3, Tg, Tgn
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-189R
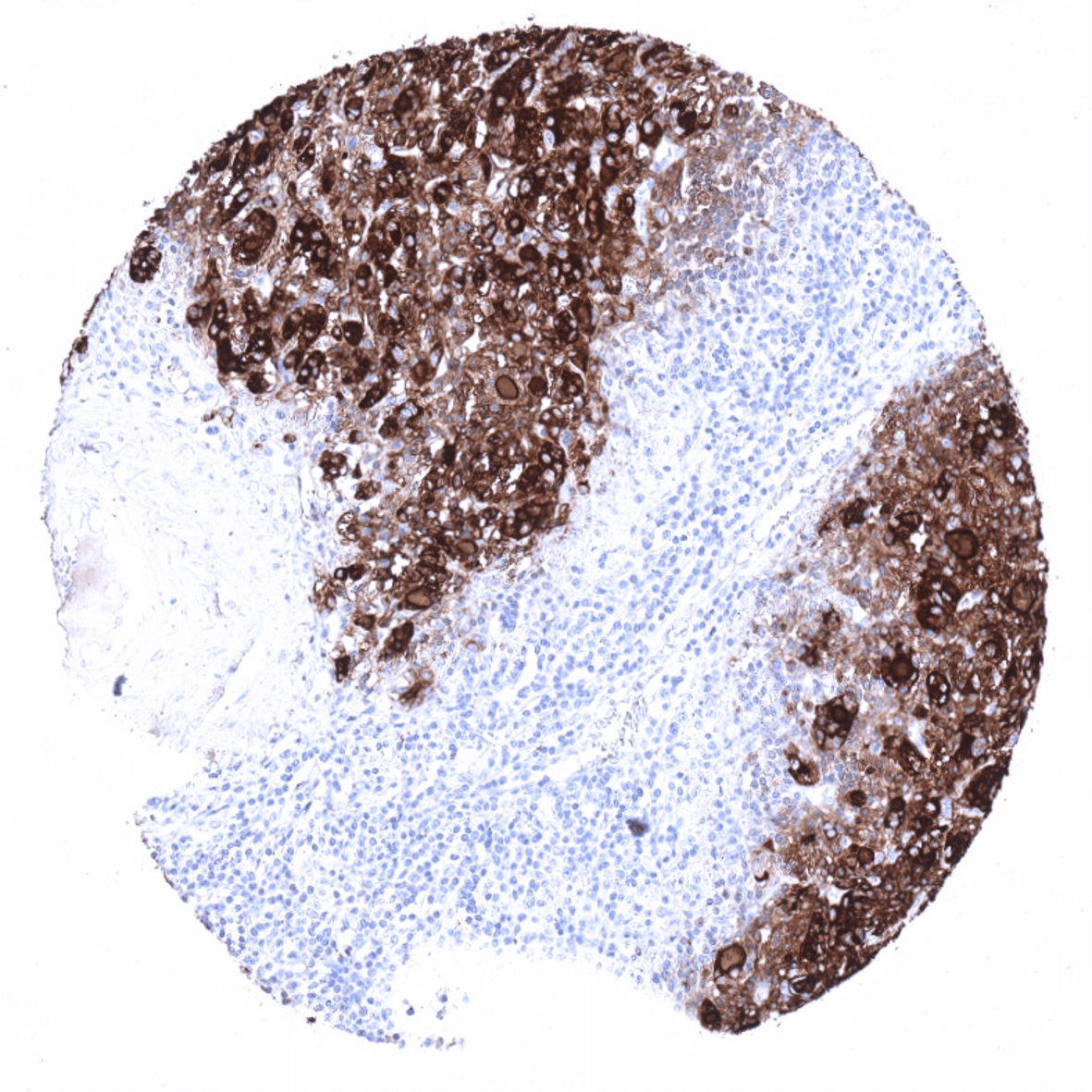
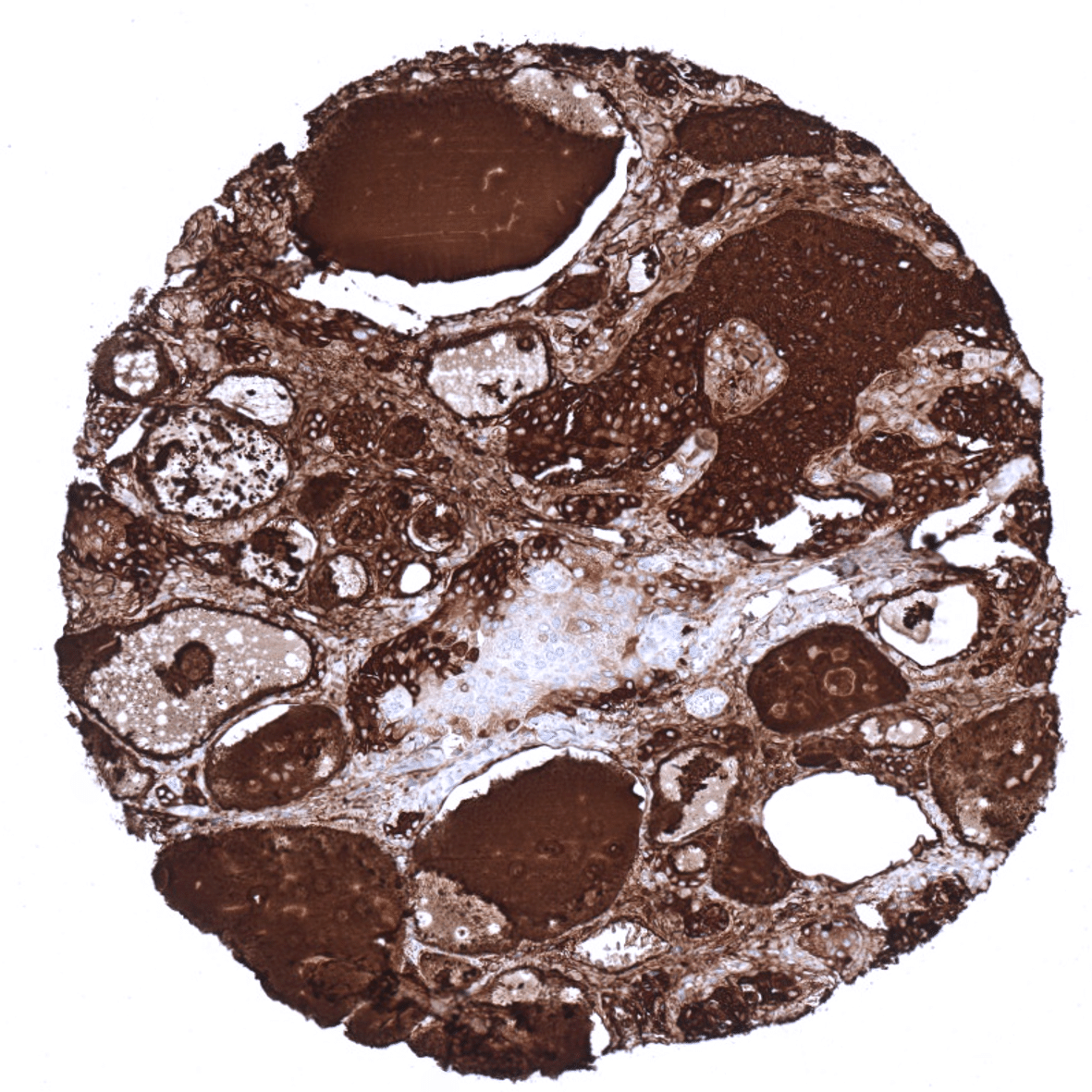
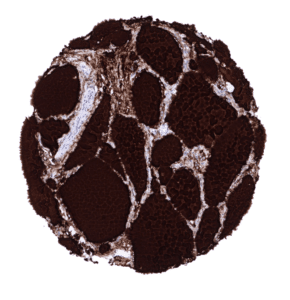
Positive control = Thyroid: A strong staining is seen in follicular cells and the colloid. The stroma is typically also stained due to a contamination artifact.
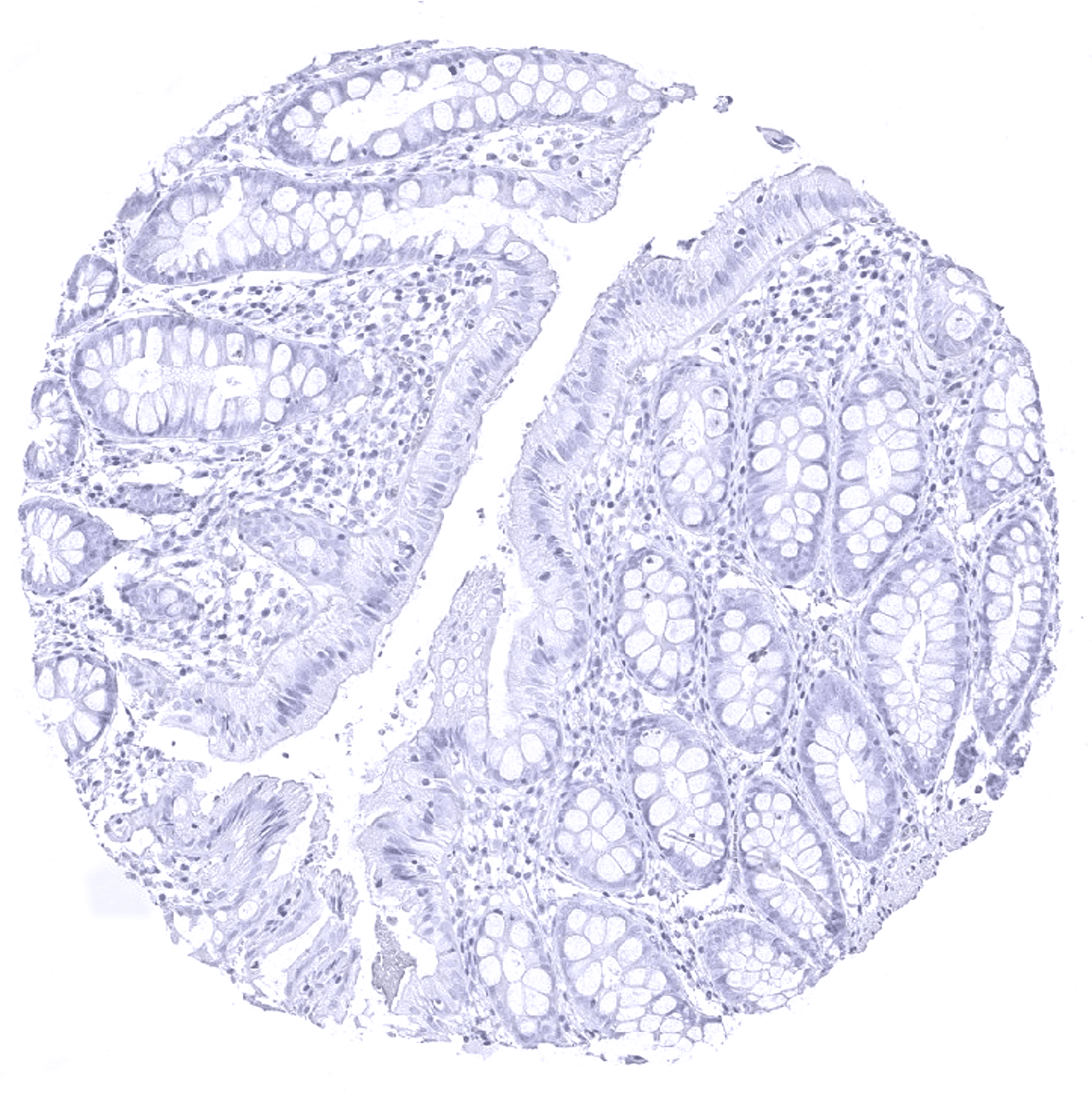
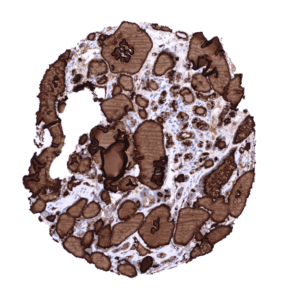
Negative control = Colon: All cells must not show any staining.
Cellular localization = Cytoplasmic
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
Biology Behind
Thyroglobulin is a 660 kDa glycoprotein encoded by the TG gene located at human chromosome 8q24.22. The glycoprotein is predominantly expressed by follicular cells of the thyroid gland and assembles into homodimers which are secreted into the follicular lumen. Thyroglobulin is the substrate for the synthesis of thyroxine and triiodothyronine, two major hormones of the thyroid gland, and serves as a storage protein for the inactive forms of these hormones in the follicular colloid. Polymorphisms of the TG gene have been linked to susceptibility of autoimmune thyroid diseases including Hashimoto thyroiditis.[1]
[1] Steurer et al.: “Immunohistochemically detectable thyroglobulin expression in extrathyroidal cancer is 100% specific for thyroidal tumor origin”. Published in Annals of Diagnostic Pathology, Volume 54, October 2021, 151793
Staining Pattern in Normal Tissues
In normal tissues, thyroglobulin immunostaining is exclusively seen in thyroid tissue. In the thyroid, immunostaining often not only involves follicular epithelial cells but also stroma and other cell types. This is due to the abundance of thyreoglobulin in the thyroid. Thyroglobulin makes up for about half of the protein mass of the thyroid. In case of immunostaining, some thyroglobulin spill-over occurs resulting in a staining of follicle-adjacent tissues.
These findings are largely comparable to the data described in the Human Protein Atlas (Tissue expression Thyroglobulin).
Suggested positive tissue control: Thyroid: A strong staining is seen in follicular cells and the colloid. The stroma is typically also stained due to a contamination artifact.
Suggested negative tissue control: Colon: All cells must not show any staining.
Staining Pattern in Relevant Tumor Types
Thyroglobulin expression is only found in tumors derived from the thyroid and in metastases from extrathyroidal tumors to the thyroid. In the case of metastases to the thyroid, tissue contamination by colloid from potentially destructed adjacent follicles is likely to cause unexpected positivity in a fraction of cases. For the same reason thyroglobulin immunostaining can also be seen in medullary (or anaplastic) carcinomas of the thyroid, which do not express thyroglobulin.
Detailed data on Thyroglobulin staining by MSVA-189R obtained from an analysis of >9000 tumors from 109 different tumor types and subtypes have recently been published Steurer et al.: “Immunohistochemically detectable thyroglobulin expression in extrathyroidal cancer is 100% specific for thyroidal tumor origin”. – Detailed data below “Compatibility of Antibodies”
The TCGA findings on Thyroglobulin RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
Thyroglobulin (MSVA-189R) publication summary
Paper used for data compilation: Steurer et al.: “Immunohistochemically detectable thyroglobulin expression in extrathyroidal cancer is 100% specific for thyroidal tumor origin”. Published in Annals of Diagnostic Pathology, Volume 54, October 2021, 151793
In this study, a total of 9974 tumors were analyzed from 109 different tumor categories by using the following protocol: Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 6 Target Retrieveal Solution buffer. MSVA-189R at a dilution of 1:135 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent). This protocol was also used for all stainings depicted in our tumor and normal tissue galleries.
The distribution of positive staining results is shown in an “organ-systematic” and in a “ranking order” figure below (images based on a compilation of data from Steurer et al.). A positive thyroglobulin immunostaining was only seen in tumors located in the thyroid. Among these, almost all follicular adenomas (99,1%), papillary carcinomas (98,1%), and follicular carcinomas (95,2%) were thyroglobulin positive. Of note, some thyroglubulin staining was also recorded in 42,4% of medullary and 5% of 40 anaplastic thyroid carcinomas as well as in one diffuse large B-cell lymphoma located in the thyroid. While these tumors are known to not express thyroglobulin, the authors concluded that these positive staining results were due to contamination artifacts caused by the very high quantities of thyroglobulin in adjacent normal follicles.
Authors conclusions on diagnostic utility with respect to the distinction of benign versus malignant (Jansen et al.)
- none
Authors conclusions on diagnostic utility with respect to the distinction of different tumor entities (Steurer et al.):
- In tumors not located in the thyroid, a positive thyroglobulin staining is near to 100% specific for a thyroidal origin.
- In tumors within the thyroid, a positive thyroglobulin staining is not completely specific for a thyroidal origin because contamination artifacts occur in the thyroid.
Authors conclusions on prognostic/predictive role of thyroglobulin expression (Steurer et al.):
- none
Data from the publication: “Immunohistochemically detectable thyroglobulin expression in extrathyroidal cancer is 100% specific for thyroidal tumor origin”. Published by Steurer et al. in Annals of Diagnostic Pathology, Volume 54, October 2021, 151793
Summarized in own graphics:
- Thyroglobulin staining in tumors “organ-specific” with antibody MSVA-189R
2. Thyroglobulin staining in tumors “ranking order” by positivity with antibody MSVA-189R.
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut TMA sections were deparaffinized and exposed to heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 6 Target Retrieval Solution buffer. Apply MSVA-189R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Omnis
Unmasking IHC EnV FLEX TRS High pH 97°C ; FLEX peroxidase blocking for 3 minutes (32°C), MSVA-189R 1:150 for 30 minutes at 32°C, FLEX+ mouse/rabbit (LINKER) for 10 minutes at 32°C, horseradish peroxidase (HRP) for 30 minutes (32°C), FLEX DAB+Sub-Chromo for 5 minutes (32°C), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-189R 1:150 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-189R 1:150 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Roche – Ventana Discovery ULTRA
Pretreatment for 64 minutes at 100°C (pH 8,4); CM peroxidase blocking for 12 minutes (room temperature), MSVA-189R 1:150 for 20 minutes at 36°C, secondary antibody (anti-mouse HQ) for 12 minutes at 36°C, anti-HQ HRP for 12 minutes at room temperature, DAB at room temperature, hematoxylin II at room temperature for 8 minutes, bluing reagent at room temperature for 4 minutes.
These images depict staining results obtained by the protocol described above. It is of note, that the Ventana machines generally require higher antibody concentrations than other commonly used autostainers because the antibodies are automatically diluted during the procedure. Various other protocols can result in an identical result as shown above. A longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of secondary antibody and or the anti-HQ HRP result in stronger staining, potentially at the cost of more background staining.
Potential Research Applications
No data available at the moment.
Evidence for Antibody Specificity in IHC
Specificity of MSVA-189R is documented by strong positive staining thyroid follicular cells in combination with complete absence of any thyroglobulin immunostaining in any other normal tissues.