295,00 € – 995,00 €
Product details
Synonyms = BCEI, Breast Cancer Estrogen Inducible Protein, Gastrointestinal Trefoil Protein, Gastrointestinal trefoil protein pS2, HP1A, HPS2, pNR2, TFF1, Trefoil Factor 1
Antibody type = Mouse monoclonal / IgG
Clone = MSVA-482M
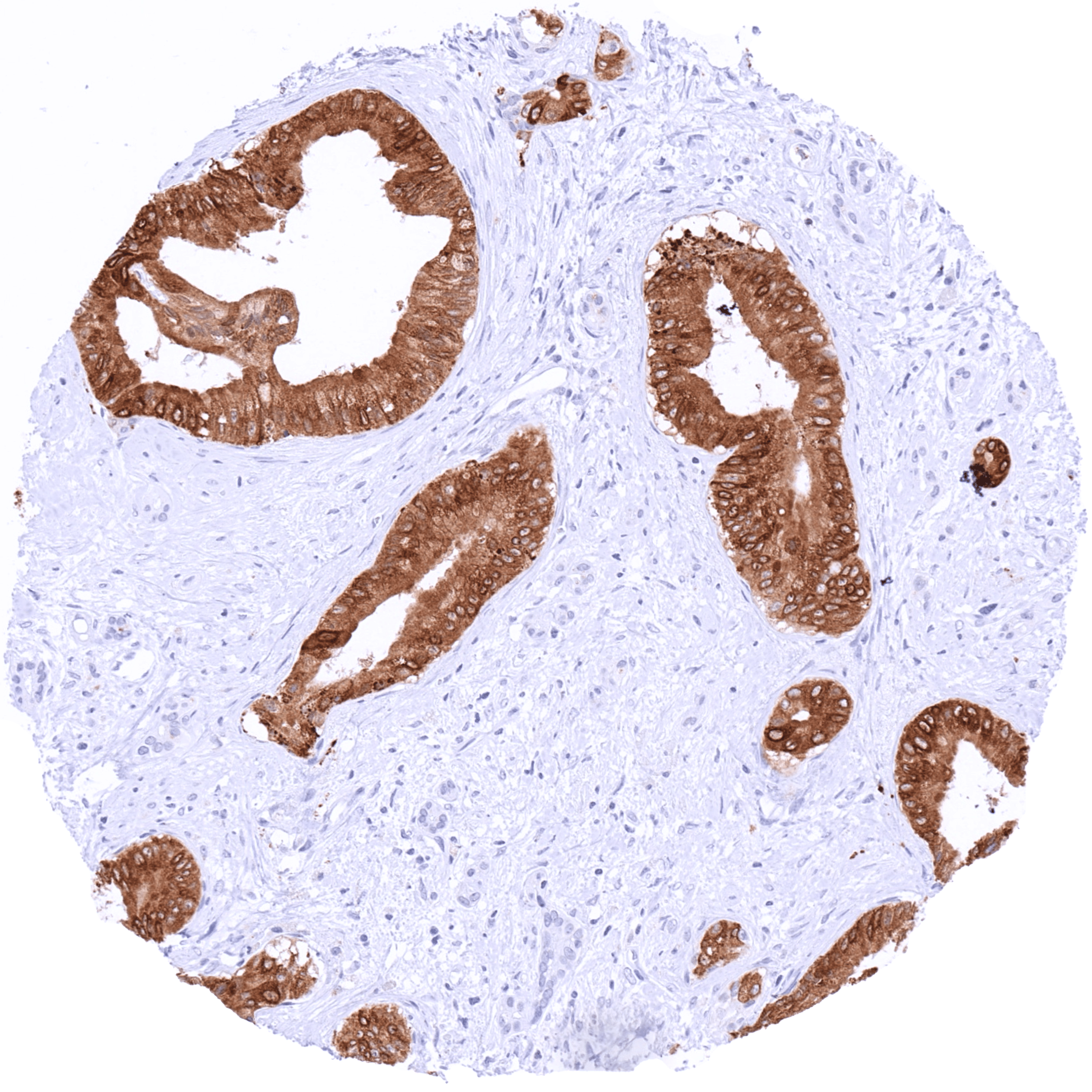
Positive control = Stomach: A strong cytoplasmic TFF1 staining should be seen in the gastric surface epithelium.
Negative control = Kidney: TFF1 immunostaining should be absent in all cell types.
Cellular localization = Cytoplasm
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
TFF1 is an essential molecule in the mucus barrier of the stomach.
Biology Behind
Trefoil factor 1 (TFF1) is a highly conserved secreted protein with a molecular weight of 6.5kDa. It is coded by the TFF1 gene located at 21q22. TFF1 has lectin activities and is preferentially expressed in the gastric mucosa where it exerts a protecting effect for the mucus barrier. TFF1 plays an important role in epithelial wound repair. Its expression is partly regulated by inflammatory mediators. TFF1 has been suggested to represent a tumor suppressor gene for the stomach because somatic TTF1 mutations have been found in gastric cancers. Tff1-deficient mice serve as a gastric cancer model because 100% of them develop adenomas in the gastric mucosa and of which about 30% progress to carcinomas.
Staining Pattern in Normal Tissues
Images describing the TFF1 staining pattern in normal tissues obtained by the antibody MSVA-482M are shown in our “Normal Tissue Gallery”.
| Brain | Cerebrum | Negative. |
| Cerebellum | Negative. | |
| Endocrine Tissues | Thyroid | Negative. |
| Parathyroid | Negative. | |
| Adrenal gland | Negative. | |
| Pituitary gland | Negative. | |
| Respiratory system | Respiratory epithelium | Goblet cells in respiratory epithelium and in bronchial glands can show weak to moderate cytoplasmic TFF1 positivity. |
| Lung | Negative. | |
| Gastrointestinal Tract | Salivary glands | TFF1 positivity of varying intensity is often (but not always) seen in mucinous glands. The apical membranes of excretory ducts can also stain (may be due to secreted TFF1 positive materials). |
| Esophagus | Negative. | |
| Stomach | Very intense cytoplasmic TFF1 staining of surface epithelial cells but not of the stomach glands. | |
| Colon | Moderate cytoplasmic TFF1 staining of a small subset of goblet cells. | |
| Duodenum | Moderate to strong TFF1 staining of selected goblet cells, predominantly at the surface (villi) or the top third of the crypts. | |
| Rectum | Moderate TFF1 staining of selected goblet cells, predominantly at the surface (villi) or the top third of the crypts. | |
| Small intestine | Moderate TFF1 staining of a subset of goblet cells. | |
| Liver | Negative. | |
| Gallbladder | Weak focal cytoplasmic TFF1 staining of surface epithelial cells can occur. | |
| Pancreas | Negative. | |
| Genitourinary | Kidney | Negative. |
| Urothelium | A weak cytoplasmic TFF1 staining can be seen in some umbrella cells. | |
| Male genital | Prostate | Negative. |
| Seminal vesicles | Negative. | |
| Testis | Negative. | |
| Epididymis | Negative. | |
| Female genital | Breast | Luminal breast epithelial cells can show weak to moderate TFF1 positivity. Glands may contain TFF1 positive mucus. |
| Uterus, myometrium | Negative. | |
| Uterus, ectocervix | Negative. | |
| Uterus endocervix | Negative. | |
| Uterus, endometrium | Negative. | |
| Fallopian Tube | Negative. | |
| Ovary | Negative. | |
| Placenta early | Negative. | |
| Placenta mature | Negative. | |
| Amnion | Negative. | |
| Chorion | Negative. | |
| Skin | Epidermis | Negative. |
| Sebaceous glands | Negative. | |
| Muscle/connective tissue | Heart muscle | Negative. |
| Skeletal muscle | Negative. | |
| Smooth muscle | Negative. | |
| Vessel walls | Negative. | |
| Fat | Negative. | |
| Stroma | Negative. | |
| Endothelium | Negative. | |
| Bone marrow/lymphoid | Bone marrow | Negative. |
| Lymph node | Negative. | |
| Spleen | Negative. | |
| Thymus | Negative. | |
| Tonsil | Negative. | |
| Remarks |
These findings are fully consistent with the RNA data described in the Human Protein Atlas (Tissue expression TFF-1).
Positive control = Stomach: A strong cytoplasmic TFF1 staining should be seen in the gastric surface epithelium.
Negative control = Kidney: TFF1 immunostaining should be absent in all cell types.
Staining Pattern in Relevant Tumor Types
RNA expression screening studies have described elevated levels of TFF1 in gastric, colorectal, breast, pancreatic, and lung cancer.
The TCGA findings on TFF1 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
TFF1 (MSVA-482M) publication summary
Relevant publication: Lutz et al. “Expression of Trefoil Factor 1 (TFF1) in Cancer: A Tissue Microarray Study Involving 18’878 Tumors” Published in Diagnostics (Basel). 2024 Sep 28 PMID: 39410561
A total of 16’817 tumors from 149 different tumor categories were successfully analyzed by using the following protocol: Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. MSVA-482M, at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent). This protocol was also used for all stainings depicted in our tumor and normal tissue galleries.
Overall, 65 of 149 tumor categories showed detectable TFF1 staining with 32 tumor categories showing at least one strongly positive case. The highest positivity rates occurred in mucinous carcinomas of the ovary (76.2%), colorectal adenomas and adenocarcinomas (47.1–75.0%), tumors of the breast (up to 72.9%), biliopancreatic adenocarcinomas (42.1–62.5%), gastro-esophageal adenocarcinomas (40.4–50.0%), neuroendocrine neoplasms (up to 45.5%), cervical adenocarcinomas (39.1%), and in urothelial neoplasms (5.5–24.3%). The TFF1 positivity rates are shown in an “organ-systematic” (Figure 1) and in a “ranking order” figure (Figure 2) below (images based on data from Lutz et al). Associations with clinic-pathological parameters are also summarized (Figure 3; based on data described by Lutz et al).
Authors conclusions on diagnostic utility of TFF1 IHC with respect to the distinction of different tumor entities (Lutz et al.):
- -Given that TFF1 is expressed in a broad range of tumor entities, TFF1 IHC may have only limited utility for the discrimination of different tumor entities.
Authors conclusions on the possible clinical role of TFF1 staining (Lutz et al.):
- Association with low grade of malignancy (p = 0.0003), estrogen and progesterone receptor expression (p < 0.0001), non-triple negativity (p = 0.0005) in invasive breast cancer of no special type
- Because of its upregulation in multiple tumor entities, TFF1 may represent a suitable therapeutic target.
1. TFF1 staining in tumors “organ-specific” with antibody MSVA-482M
2. TFF1 staining in tumors “ranking order” by positivity with antibody MSVA-482M.
3. Table Clinico-pathological associations described by Lutz et al. (p-value)
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply MSVA-482M at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Potential Research Applications
- The diagnostic role of TFF1 immunohistochemistry remains to be determined.
- The prognostic role of TFF1 expression in different subtypes of cancer remains to be determined.
- The functional effects of TFF1 remain to be elucidated in cancer and in inflammation.
Evidence for Antibody Specificity in IHC
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. Comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
Orthogonal validation: For the antibody MSVA-482M specificity is suggested by the strong concordance of the immunostaining data with data from three independent RNA screening studies, including the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project, which are all summarized in the Human Protein Atlas (Tissue expression TFF-1). In these databases TFF1 RNA expression was only described to occur in the stomach, the organ for which the most extensive and the strongest TFF1 staining is observed by MSVA-482M. Distinct TFF1 IHC positive subpopulations are also seen in small intestine (subset of goblet cells), colorectum (subset of goblet cells), respiratory epithelium (some goblet cells), salivary glands (subset of mucinous glandular cells), bronchi (subset of mucinous glandular cells in in bronchial glands), breast gland (subset of luminal epithelial cells), urothelium (some umbrella cells), and gall bladder epithelium (some individual cells). In these organs TFF1 RNA expression has not been described.
Comparison of antibodies: True expression of TFF1 all tissues for which TFF1 immunostaining is found despite “absence of RNA expression (small intestine, colorectum, respiratory epithelium, salivary glands, bronchi, breast, urothelium, and gallbladder) is corroborated by comparison with a commercially available independent second antibody (termed “validation antibody”). Most likely, TFF1 positive cells represent such small subpopulations of the respective organs, that TFF1 RNA cannot be detected because of too extensive dilution if RNA is extracted from total organs.