295,00 € – 995,00 €
Product details
Synonyms = CDK4 inhibitor p16 INK4; CDK4I; CDKN2A; Cell cycle negative regulator beta; CMM2; Cyclin dependent kinase 4 inhibitor A; Melanoma p16 inhibits CDK4; MLM; MTS1; Multiple tumor suppressor 1; p14; p16; p19; P19ARF; TP16
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-016R
Positive control = Pancreas: at least a moderate staining is expected in islets of Langerhans
Negative control = Pancreas: staining should be absent in acinar cells, Normal cervix uteri: p16 staining should be absent in epithelial cells (in some cases, few cells may show weak staining)
Cellular localization = Nuclear and Cytoplasmic
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
Biology Behind
P16 is a tumor suppressor protein, which is up-regulated under several pathological conditions. The p16 protein is encoded by the cyclin dependent kinase inhibitor 2A gene (CDKN2A, syn. MTS-1, INK4a or p16INK4 located at chromosome 9p21. P16 inhibits cell cycle progression from G1 to S phase through binding and inactivating cyclin dependent kinases CDK4 and CDK6. p16 acts in concert with the retinoblastoma (RB1) and the p53 tumor suppressor genes to control cell cycle progression. In case of an inactivation of p53 or RB1, and especially in case of inactivation of both proteins, p16 can be markedly upregulated. For example, human papilloma virus (HPV) infected cells display strong p16 upregulation in an effort to compensate HPV-induced inhibition of both p53 or RB1.
Staining Pattern in Normal Tissues
P16 staining pattern in Normal Tissues with antibody MSVA-016R (images are shown in our “Normal Tissue Gallery”)
| Brain | Cerebrum | Negative. |
| Cerebellum | Negative. | |
| Endocrine Tissues | Thyroid | Usually negative. |
| Parathyroid | Usually negative. | |
| Adrenal gland | A usually weak (or moderate) p16 staining can occur in individual cells or small groups of cells (not all samples). | |
| Pituitary gland | Moderate to strong p16 staining of a large fraction of epithelial cells in the adenohypophysis. | |
| Respiratory system | Respiratory epithelium | A usually weak (or moderate) p16 staining can occur in individual cells or small groups of cells (not all samples). |
| Lung | Usually negative. | |
| Gastrointestinal Tract | Salivary glands | A usually weak (or moderate) p16 staining can occur in individual cells or small groups of cells of excretory ducts (not all samples). |
| Esophagus | A usually weak (or moderate) p16 staining can occur in individual cells or small groups of cells (not all samples). | |
| Stomach | A usually weak (or moderate) p16 staining can occur in individual cells or small groups of cells (not all samples). | |
| Duodenum | A usually weak (or moderate) p16 staining can occur in individual cells or small groups of cells (not all samples). | |
| Small intestine | A usually weak (or moderate) p16 staining can occur in individual cells or small groups of cells (not all samples). | |
| Appendix | A usually weak (or moderate) p16 staining can occur in individual cells or small groups of cells (not all samples). | |
| Colon | A usually weak (or moderate) p16 staining can occur in individual cells or small groups of cells (not all samples). | |
| Rectum | A usually weak (or moderate) p16 staining can occur in individual cells or small groups of cells (not all samples). | |
| Liver | Usually negative. | |
| Gallbladder | Usually negative. | |
| Pancreas | Weak to moderate p16 staining in a subset of cells in islets of Langerhans. | |
| Genitourinary | Kidney | Usually negative. |
| Urothelium | A usually weak (or moderate) p16 staining can occur in individual cells or small groups of urothelial cells (not all samples). | |
| Male genital | Prostate | A usually weak (or moderate) p16 staining can occur in individual cells or small groups of epithelial cells (not all samples). This mostly involves basal cells. |
| Seminal vesicles | Usually negative. | |
| Testis | Usually negative. | |
| Epididymis | Usually negative. | |
| Female genital | Breast | A usually weak (or moderate) p16 staining can occur in individual luminal cells or small groups of luminal cells (not all samples). |
| Uterus, myometrium | Negative. | |
| Uterus, ectocervix | A usually weak (or moderate) p16 staining can occur in individual cells or small groups of cells (not all samples). | |
| Uterus endocervix | Usually negative. | |
| Uterus, endometrium | Selected glands can show weak, moderate or strong p16 staining. | |
| Fallopian Tube | Usually negative. | |
| Ovary | Usually negative. | |
| Placenta early | Usually negative. | |
| Placenta mature | Stroma cells can stain p16 positive (weak to moderate), but not in all samples. | |
| Amnion | Usually negative. | |
| Chorion | Usually negative. | |
| Skin | Epidermis | Usually negative. |
| Sebaceous glands | Usually negative. | |
| Muscle/connective tissue | Heart muscle | Negative. |
| Skeletal muscle | Negative. | |
| Smooth muscle | Negative. | |
| Vessel walls | Negative. | |
| Fat | Negative. | |
| Stroma | Positive p16 staining can occur in some fibroblasts. | |
| Endothelium | A variable (sometimes strong) p16 positivity can rarely be seen in endothelial cells. | |
| Bone marrow/ lymphoid tissue | Bone marrow | Usually negative. |
| Lymph node | Weak p16 staining in some macrophages of germinal centres. Scattered p16 positive lymphocytes do occur. | |
| Spleen | Scattered p16 positive lymphocytes do occur. | |
| Thymus | Some squamous epithelial cells of corpuscles of Hassall’s stain p16 positive. Scattered p16 positive lymphocytes do occur. | |
| Tonsil | Weak p16 staining in some macrophages of germinal centres. Scattered p16 positive lymphocytes do occur. A weak or moderate p16 staining can occur in individual cells or small groups of crypt and/or surface epithelium. | |
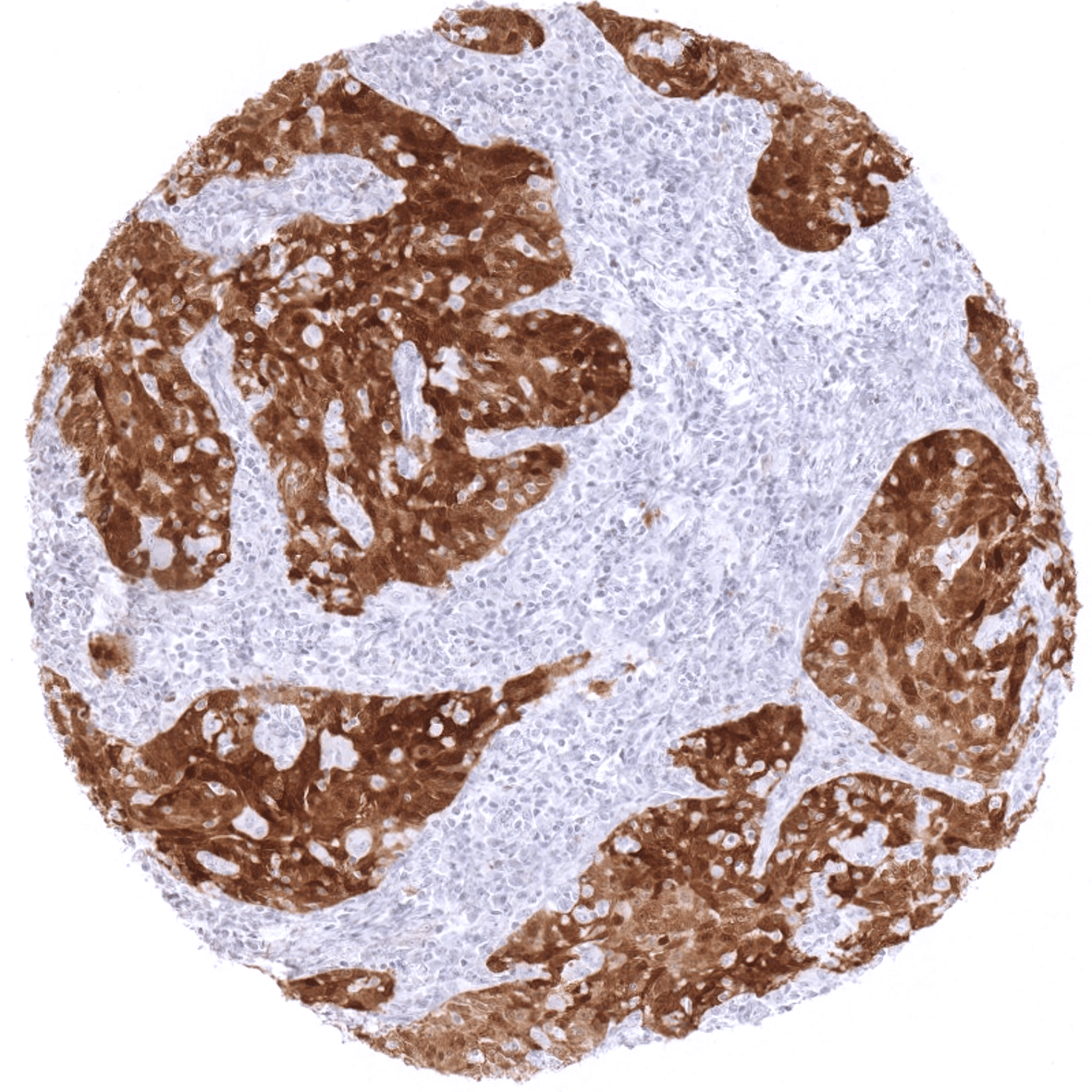
| Remarks | In principle, at least a subtle p16 (cytoplasmic and nuclear) staining can occasionally occur in every normal tissue. P16 staining is strongest in islet cells of the pancreas and epithelial cells of the adenohypophysis. |
These findings are largely consistent with the RNA data described in the Human Protein Atlas (Tissue expression p16). RNA data from 2 of 3 referenced RNA datasets (GTEx and FANTOM5) describe highest RNA levels in the pituitary gland and low expression in the vast majority of other tissues. The third dataset (HPA) lacks pituitary gland data but also describes low expression in all tissues but T-lymphocytes. The strong RNA expression in testis described in the GTEx database is not detectable by MSVA-016R. This fits with the FANTOM5 and HPA datasets also not describing elevated p16 RNA expression in testis. The protein data described in the protein atlas are inconsistent with MSVA-016R findings. However, from three antibodies used for collecting the protein atlas data, two (CAB000093, CAB018232) did not show staining across all tissues, and the third (CAB000445) considered positive in most tissues regularly showed staining patterns consistent with non-specificity such as granular perinuclear staining.
Suggested positive tissue control: Pancreas: at least a moderate staining is expected in islets of Langerhans
Suggested negative tissue control: Pancreas (no staining in acinar cells), Normal cervix uteri (no staining, except – in some cases – occasional cells with weak positivity)
Staining Pattern in Relevant Tumor Types
The TCGA findings on p16 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
p16 (MSVA-016R) publication summary
Relevant publication: De Wispelaere et al. “High prevalence of p16 staining in malignant tumors.” Published in PLoS One. 2022 Jul 21;17(7):e0262877 PMID: 35862385.
A total of 11,759 tumors from 124 different tumor categories were successfully analyzed by using the following protocol: Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 9 Target Retrieval Solution buffer. MSVA-016R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent). This protocol was also used for all stainings depicted in our tumor and normal tissue galleries.
All 124 tumor categories showed a detectable p16 expression in at least one case and 71 (57.3%) tumor categories showed at least one case with strong positivity. The distribution of positive staining results is shown in “organ-systematic” and in “ranking order” figures below (images based on data from De Wispelaere et al.):
Authors conclusions on diagnostic utility of p16 IHC with respect to the distinction of benign versus malignant (De Wispelaere et al.):
- Because of the rarity of significant p16 staining in normal tissues, p16 positivity can be regarded as a feature for malignancy. Examples include:
- Distinction of liposarcoma (56.6% strongly positive) from lipoma (almost always negative).
- Distinction of leiomyosarcoma (21% strongly positive) from leiomyoma (almost always negative).
- Distinction of urothelial dysplasia (p16 positive in >40%) from normal urothelium (p16 positive in <10%).
Authors conclusions on diagnostic utility of p16 IHC with respect to the distinction of different tumor entities (De Wispelaere et al.):
- That a minimum of one case with at least a moderate p16 positivity was found in 100 of our 124 (80.6%) analyzed cancer types demonstrates that p16 immunostaining offers only limited support for defining a tumor’s site of origin.
- The imperfect association between p16 immunostaining and HPV infection in squamous cell carcinomas of different organs challenges the use of p16 IHC as a surrogate for HPV positivity (except in tumors of cervix uteri and the penis).
- Some utility for the distinction of high-grade endometrial carcinoma (p16 positive in 5%) from serous carcinoma of the endometrium (p16 positive in 40%).
Authors conclusions on the prognostic role of p16 immunostaining results (De Wispelaere et al.):
- p16 positivity is linked to high grade in breast cancer NST.
- p16 positivity is linked to high grade and muscle-invasion in urothelial carcinoma.
- p16 positivity (typically weak) is linked to MSI in colorectal cancer.
Data from the publication: “High prevalence of p16 staining in malignant tumors.” Published by De Wispelaere et al. in PLoS One. 2022 Jul 21;17(7):e0262877 PMID: 35862385. Summarized in own graphics:
Figure 1. p16 staining in cancer (“organ-systematic” ; according to De Wispelaere et al.)
Figure 2. p16 staining in cancer (“ranking – order” ; according to De Wispelaere et al.)
Figure 3. Clinico-pathological associations described by De Wispelaere et al. (p-value)
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in on image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH9 Target Retrieval Solution buffer. Apply MSVA-016R at a dilution of 1:150 at 37°C for 60 minutes.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-016R 1:150 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-016R 1:150 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Impact of pH
The strongest p16 staining by MSVA-016R is obtained at a pH 9,0. However, pH 7,8 results in only a slight reduction of the staining intensity as compared to pH9. We thus consider pH7,8 as optimal for manual staining because of the better tissue preservation at pH7,8 than at pH 9,0.
Potential Research Applications
- A comprehensive study analyzing p16 expression in various different tumor entities would be helpful to assess the diagnostic significance of p16 IHC.
- The prognostic role of p16 alterations (overexpression, loss of expression) is of interest.
- p16 is an important interaction partner of several relevant cancer related pathways (p53, rb. others) and could thus be investigated together with other members of these pathways for a combined clinical impact.
Evidence for Antibody Specificity in IHC
Specificity of MSVA-016R is documented by strong positive staining in cell types that are well documented to express p16 such as cells of islets of Langerhans in the pancreas and HPV infected tissues in combination with absence of staining in all tissues known to not express p16 at relevant levels such kidney and colonic mucosa both known for frequent occurrence of non-specific background staining.