Product details
Synonyms = Estrogen Receptor alpha delta 4*5,6,7*/654 isoform;Estrogen Receptor alpha delta 4 +49 isoform; Nuclear receptor subfamily 3 group A member 1
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-564R
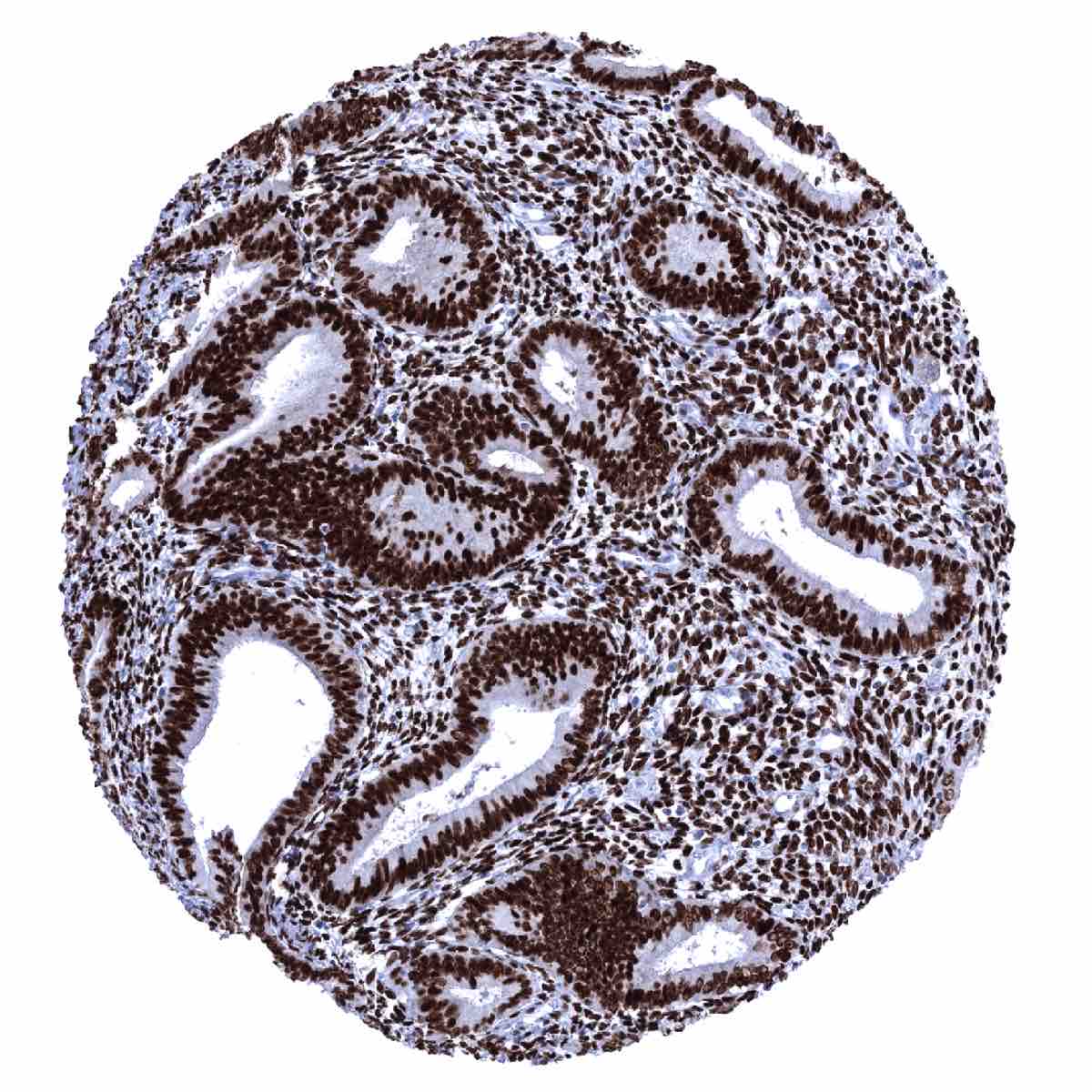
Positive control = Uterine cervix: virtually all squamous and endocervical cells must show a moderate to strong nuclear staining. Tonsil: Scattered follicular dendritic cells in germinal centers and some squamous epithelial cells in tonsil crypts must show an at least weak nuclear ER immunostaining.
Negative control = Tonsil: B-cells in mantle zones and within germinal centers must be negative.
Cellular localization = Nucleus
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100-1:200
Intended Use = Research Use Only
Relevance of Antibody
Estrogen receptor is expressed in estrogen dependent cell types.
Biology Behind
Estrogen receptor alpha (ERα) is a nuclear receptor that is coded by the gene ESR1 located at chromosome 6q25.1. ERα is a ligand-activated transcription factor composed of several domains for hormone binding, DNA binding, and activation of transcription. The classical model of ERα function is that its activation is triggered by estrogen binding to the ERα in the cytoplasm. ERα then dimerizes, translocates to the nucleus, binds to DNA and recruits the necessary transcriptional coregulators that are needed to regulate the transcription of target genes. Evidence suggests that Erα has additional – less well understood – functions that may involve the establishment of chromatin loops and nonnuclear signaling. Irrespective of the exact mechanisms, ERα plays a pivotal role in the development and function of multiple organ systems, including the reproductive (female and male), central nervous, skeletal, and cardiovascular systems. Accordingly, ERα is widely expressed throughout the body. Erα is well-established therapeutic target for various small molecules that are applied for treating breast cancer patients and for prevention of breast cancer in high-risk women.
Staining Pattern in Normal Tissues
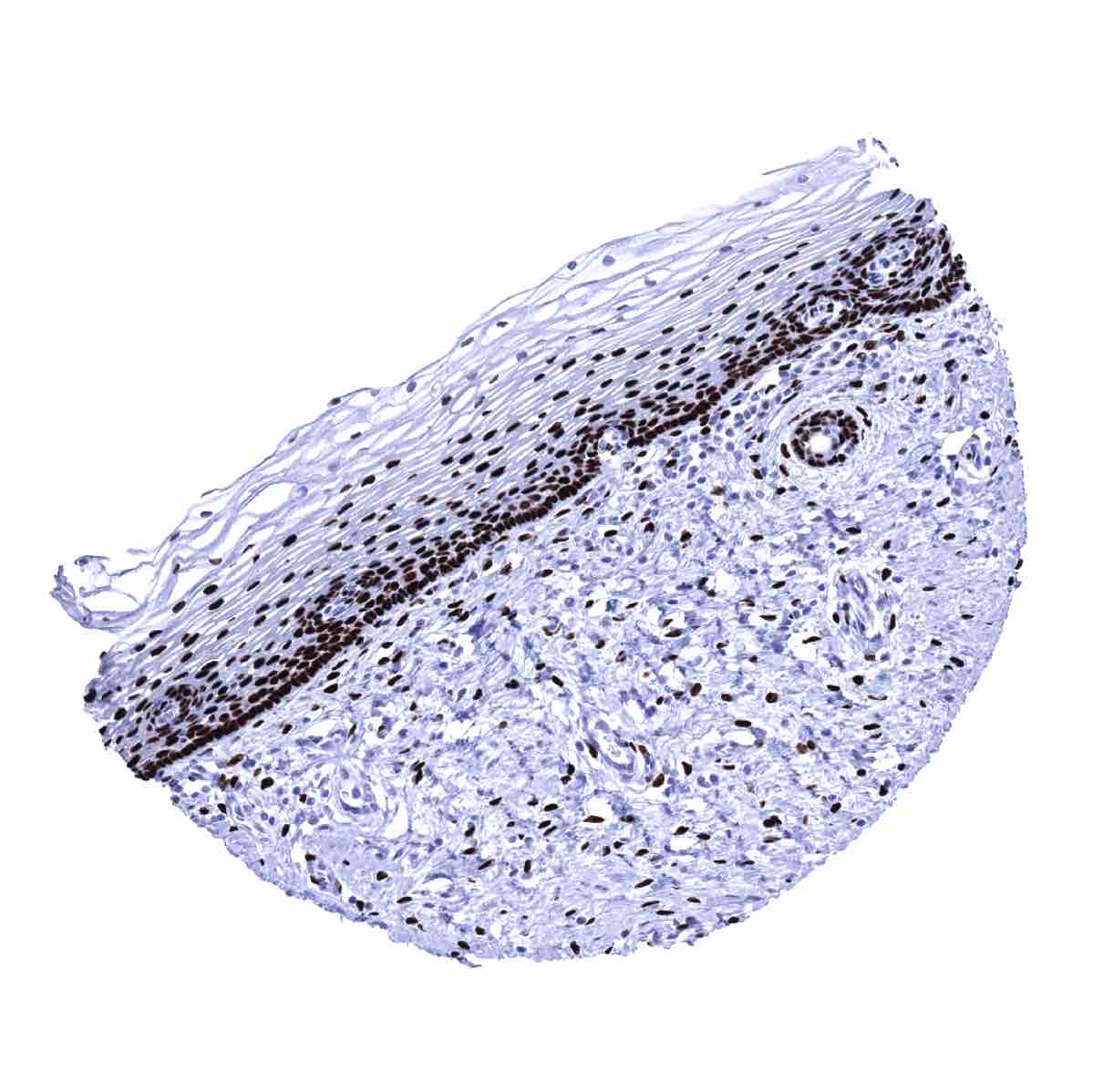
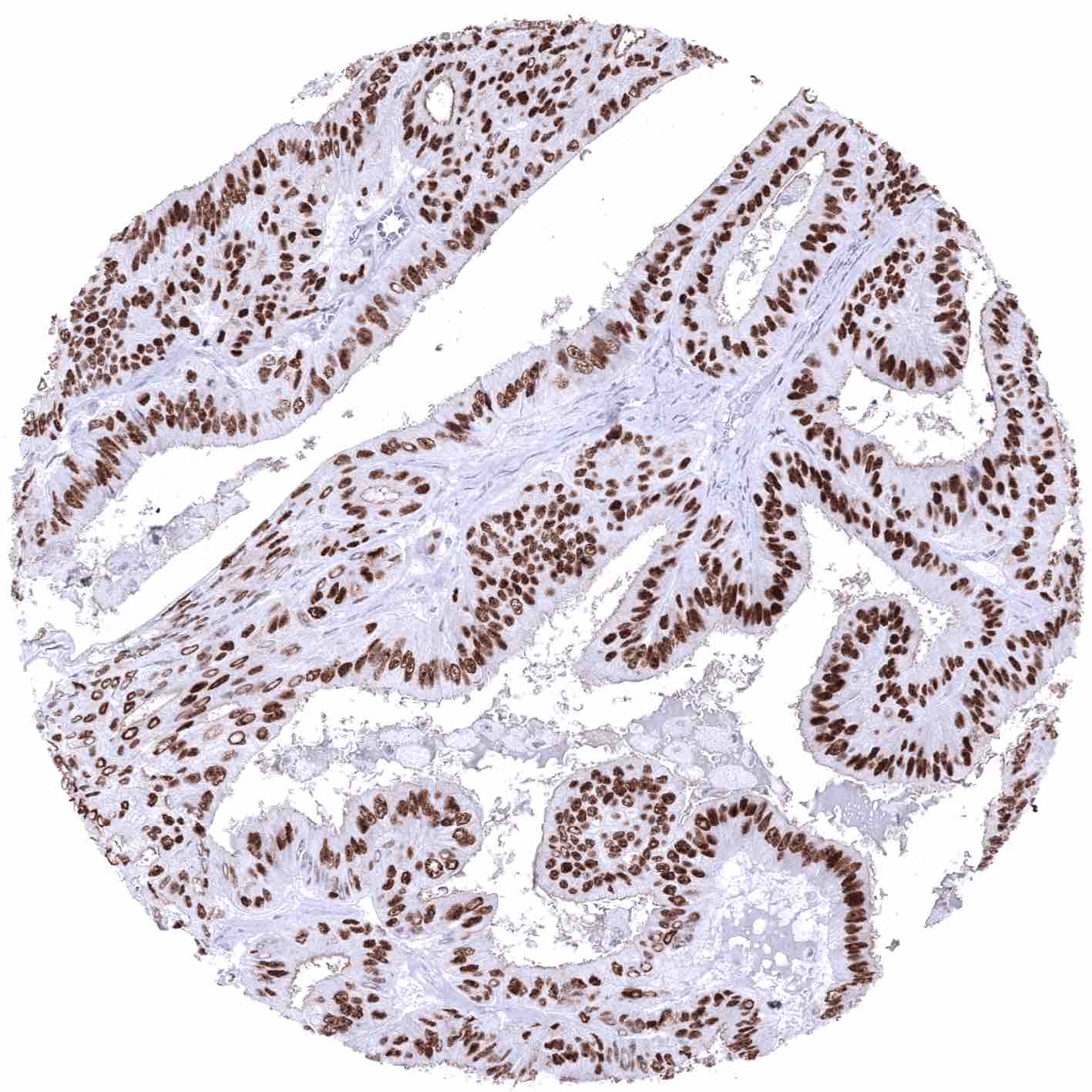
The strongest expression of estrogen receptor alpha (ER) is found in the female genital tract including decidual, epithelial and stromal cells of the endometrium and the endocervix, stromal cells and squamous epithelial cells of the ectocervix (lower 2/3), the myometrium, epithelial and stromal cells of the fallopian tube, stromal cells and corpus luteum of the ovary, and in a fraction of the epithelial cells of the breast gland. A moderate to strong ER immunostaining also occurs in stromal cells of the prostate and the seminal vesicles, epithelial cells of the cauda epididymis, peripheral germinative cell of sebaceous glands, peripheral cells of hair follicles, stroma cells of urinary bladder, as well as in a fraction (10-30%) of epithelial cells in the adenohypophysis. A variable, weak to moderate ER positivity can occasionally be seen in a fraction of adrenocortical cells, epithelial cells of salivary glands, smooth muscle cells in the aortic wall, few islet cells and some other cells of the pancreas, and a fraction of hepatocytes in some samples. In lymphatic tissues, dispersed follicular dendritic cells in germinal centers show a weak to moderate staining. In squamous epithelia, ER immunostaining is always seen in the ectocervix (strong) and in scattered cells of the tonsil epithelium (weak to moderate; crypts > surface). In squamous epithelial cells from other sites, including the skin, ER staining is usually absent but positivity of a variable number of cells can occasionally be seen. ER positivity is also seen in transitional epithelium of the anal canal (moderate) and in few urothelial cells (weak).
These findings are largely consistent with the RNA and protein data described in the Human Protein Atlas (Tissue expression Estrogen Receptor) Immunostaining by using MSVA-564R was detected in all organs with documented ERα RNA expression such as all organs of the female genital tract (except placenta), seminal vesicle, prostate, epididymis, salivary glands, liver, urinary bladder, and the pituitary gland.
Positive control = Uterine cervix: virtually all squamous and endocervical cells must show a moderate to strong nuclear staining. Tonsil: Scattered follicular dendritic cells in germinal centers and some squamous epithelial cells in tonsil crypts must show an at least weak nuclear ER immunostaining.
Negative control = Tonsil: B-cells in mantle zones and within germinal centers must be negative.
Staining Pattern in Relevant Tumor Types
The TCGA database on RNA expression in cancer has described the highest levels of ER expression in breast and endometrial cancer followed by ovarian, cervical, and thyroid cancer. Most other important tumor entities are described to be usually “ER negative”.
The TCGA findings on Estrogen Receptor RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 9 Target Retrieval Solution buffer. Apply MSVA-564R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-564R 1:100 for 40 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-564R 1:100 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Potential Research Applications
- ERα is an important drug target in breast cancer. Various new inhibitory molecules are still under development.
- Because of partly controversial data, the diagnostic utility of ER IHC should be investigated in a large cohort of tumors from different entities.
- The functional effects of plasma membrane ERα and its role in nonnuclear signaling needs to be further investigated.
- The molecular basis for tissue-type specific roles of ERα and of tissue-type specific effects of ERα inhibitors is still not fully understood.
Evidence for Antibody Specificity in IHC
There are two ways, how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are:
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
Orthogonal validation: For the antibody MSVA-564R specificity is suggested by the concordance of the immunostaining data with data from three independent RNA screening studies, including the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project, which are all summarized in the Human Protein Atlas (Tissue expression Estrogen Receptor). Immunostaining by using MSVA-564R was detected in all organs with documented RNA expression such as all organs of the female genital tract (except placenta), seminal vesicle, prostate, epididymis, salivary glands, liver, urinary bladder, and the pituitary gland.
However, using MSVA-564R, ER immunostaining can also be seen in several tissues without documented RNA expression. These tissues include peripheral germinative cell of sebaceous glands, peripheral cells of hair follicles, a fraction of adrenocortical cells, smooth muscle cells in the aortic wall, few islet cells and some other cells of the pancreas, dispersed follicular dendritic cells in germinal centers of lymphatic tissues, a small number of cells in squamous epithelia from several different sites, transitional epithelium of the anal canal and in few urothelial cells (weak). These ER positive structures constitute small subsets of the total amount of cells in these tissues/organs and may have been largely underrepresented in RNA analyses and may therefore have remained undetected.
Comparison of antibodies: True ER expression in all these cell types with ER positivity by MSVA-564R but not showing RNA expression was suggested by a comparison of the MSVA-564R staining with data obtained by an independent commercial antibody (“Validation Antibody”). All these findings were independently found by both antibodies. Images comparing MSVA-564R and the independent “validation antibody” are shown below.
Antibody Comparison: MSVA-564R vs another commercially available Estrogen Receptor antibody called “Validation Antibody”