195,00 € – 695,00 €
Product details
Synonyms = CK-14; Dowling Meara; ebs3; ebs4; Epidermolysis Bullosa Simplex; Keratin Type I Cytoskeletal 14; Koebner; NFJ
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-614R
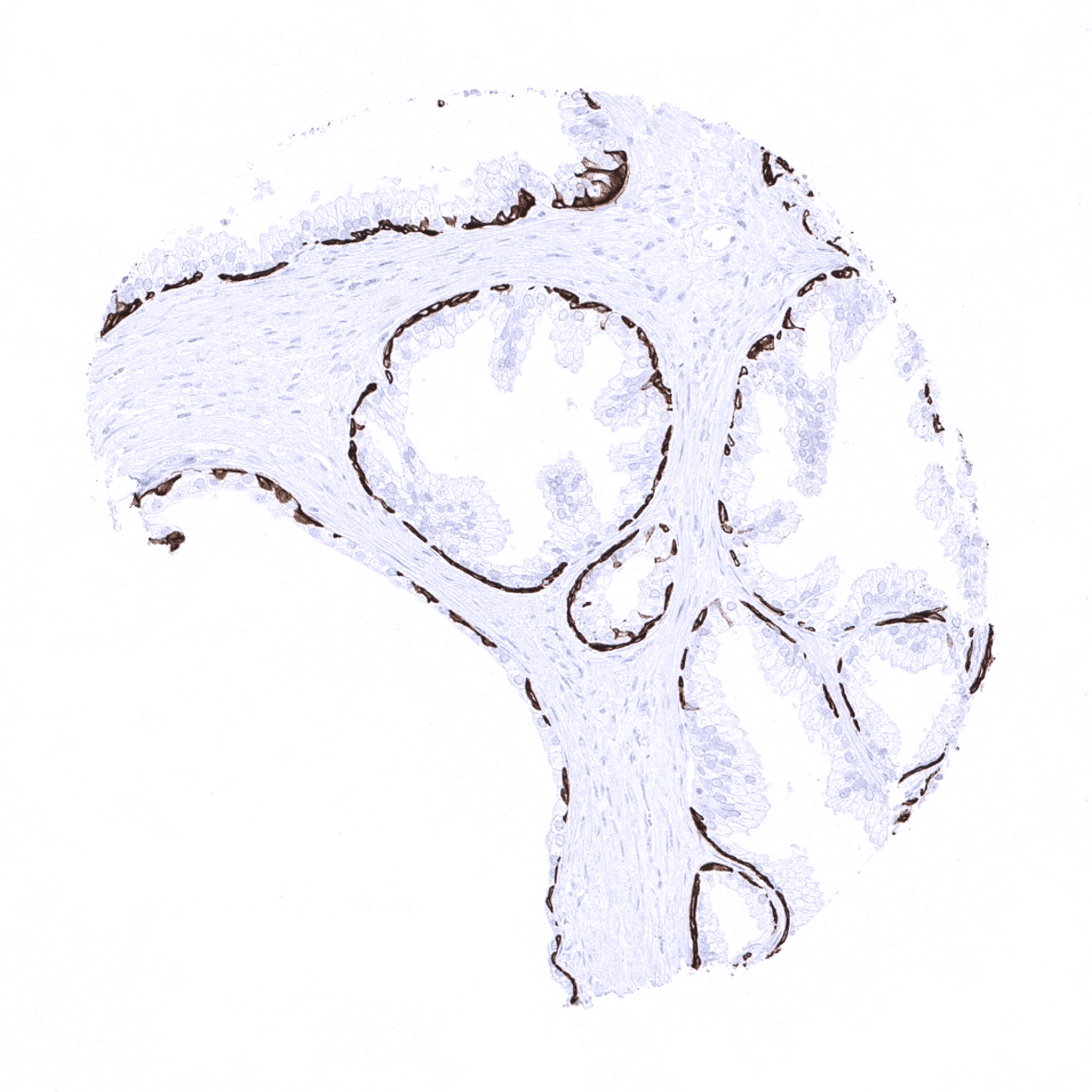
Positive control = Tonsil: A strong KRT14 immunostaining should be seen in the surface and crypt epithelium.
Negative control = Tonsil: KRT14 immunostaining should be absent in all non-epithelial cells.
Cellular localization = Cytoplasmic
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:50 – 1:100
Intended Use = Research Use Only
Relevance of Antibody
Cytokeratin 14 is expressed in basal and myoepithelial cells of the prostate and the breast.
Biology Behind
Cytokeratin 14 (CK14), also termed keratin 14 (KRT14) is a 50 kDa type I acidic keratin protein encoded by the KRT14 gene at 17q21.2. KRT14 is part of the cytoskeletal scaffold within epithelial cells, which contributes to the cell architecture and provides the cells with the ability to withstand mechanical stress. Keratin 14 is usually found as a heterodimer with type II keratin 5. Mutations in KRT14 are associated with epidermolysis bullosa simplex and dermatopathia pigmentosa reticularis.
Staining Pattern in Normal Tissues
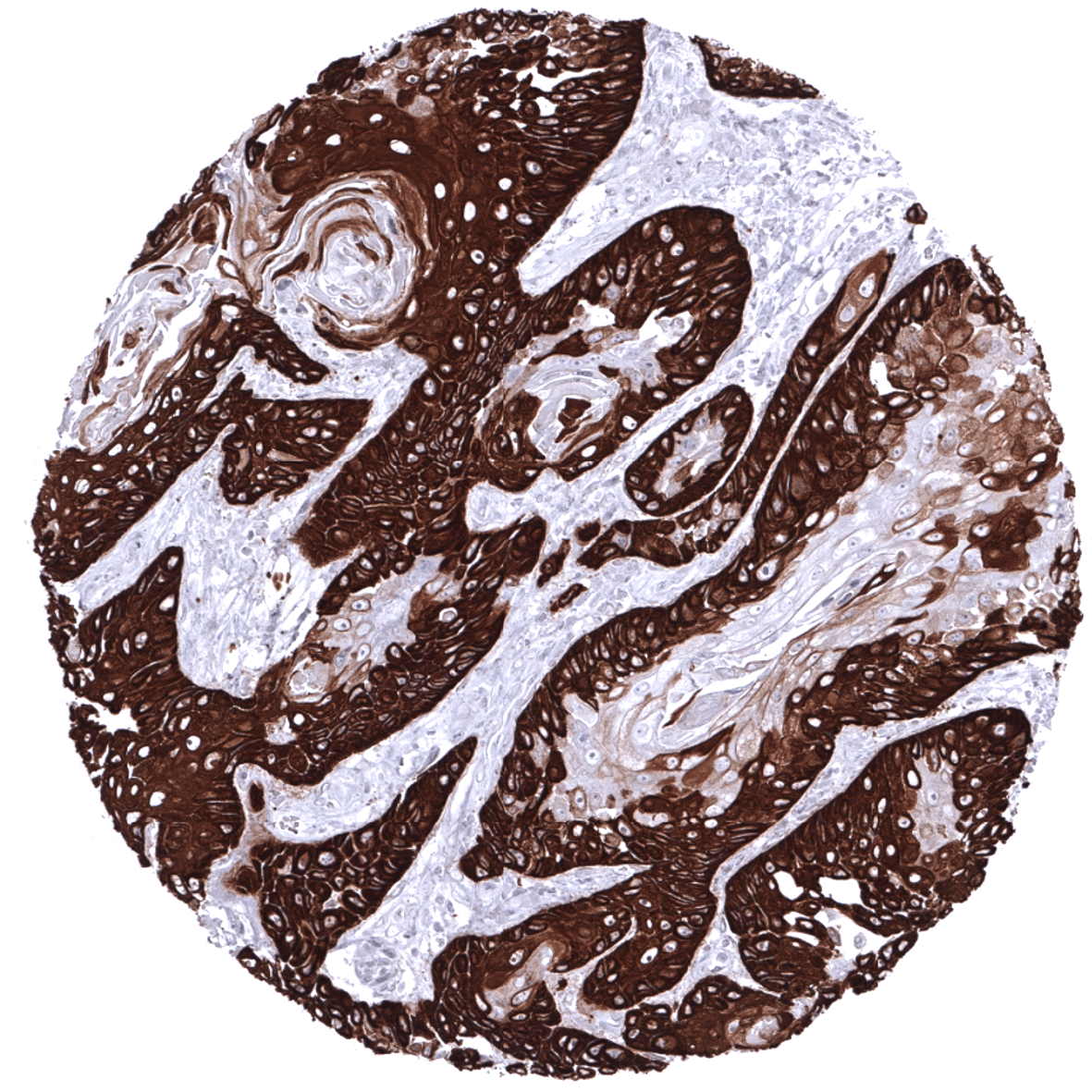
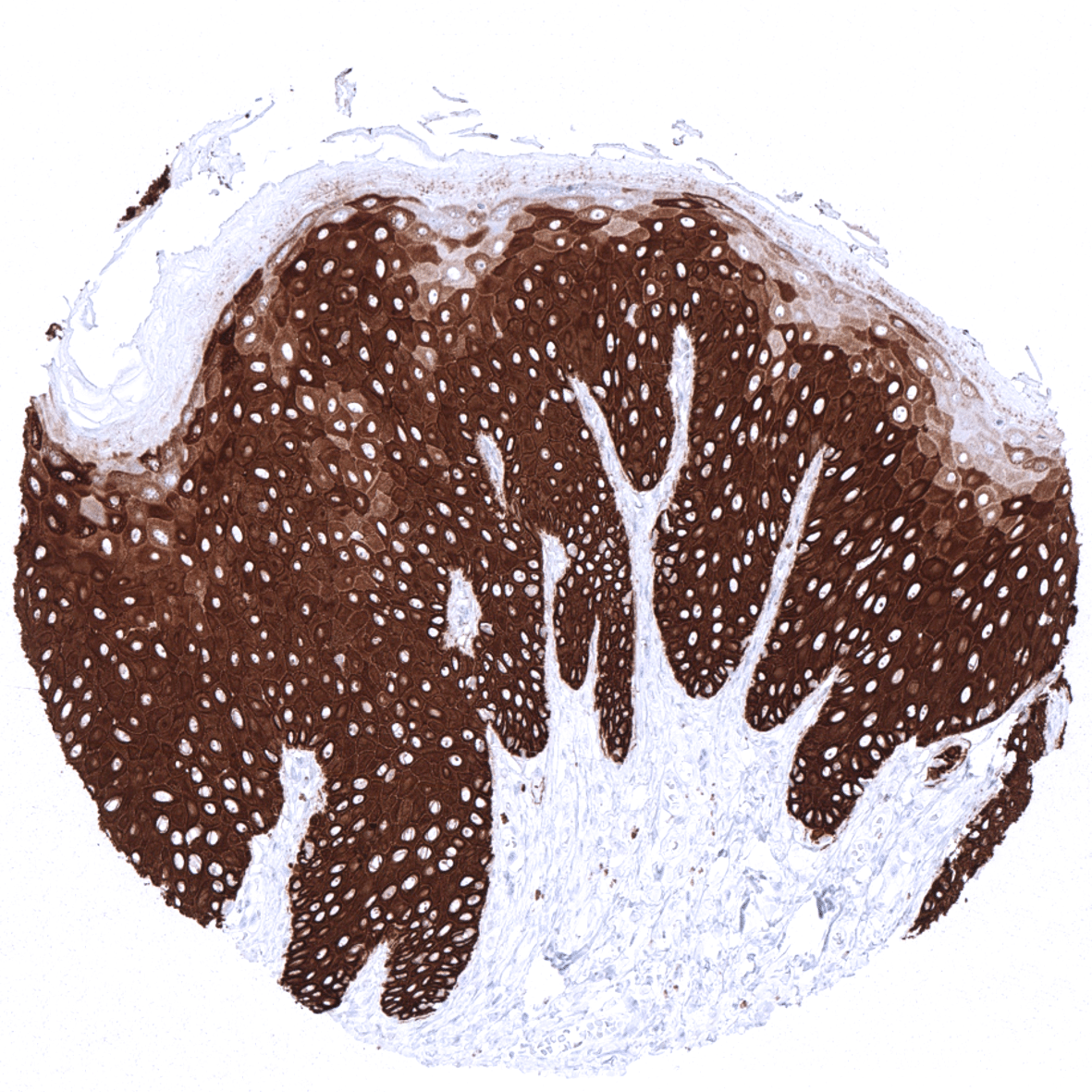
In the skin and in non-keratinizing squamous epithelium all cell layers usually show a strong KRT14 staining, but staining is sometimes weaker or even absent in the more superficial cell layers. Strong KRT14 positivity is also seen in sebaceous glands and hair follicles, epithelial cells including corpuscles of Hassall’s in the thymus, tonsil crypt epithelium, basal cells of the prostate and respiratory epithelium as well as in Amnion and chorion cells of the placenta. In salivary glands, KRT14 is strongly expressed in myoepithelial cells, basal cells and a fraction of ductal cells of excretory ducts. KRT14 immunostaining is absent in lung, liver, pancreas, epididymis, testis, urothelium, gastrointestinal epithelial cells, Brunner glands, fallopian tube, trophoblastic cells of the placenta, adrenal gland, parathyroid gland, brain, adeno- and neurohypophysis, spleen, lymph node, all hematopoetic cell types, and all mesenchymal tissues.
These findings are largely consistent with the RNA and protein data described in the Human Protein Atlas (Tissue expression Cytokeratin 14). It is of note, that RNA expression was not described for prostate and the respiratory tract, where a basal cell immunostaining is seen by MSV-614R. This basal cell staining is, however, consistent with the protein data of the protein atlas. These basal cells constitute only small subsets of the total amount of cells in these organs and may have been largely underrepresented in RNA analyses.
A faint KRT14 staining in glomeruli may represent a (tolerable) cross-reactivity of the antibody, as RNA and protein data of the protein atlas do not show such a staining.
Suggested positive tissue control: Tonsil: A strong KRT14 immunostaining should be seen in the surface and crypt epithelium.
Suggested negative tissue control: Tonsil: KRT14 immunostaining should be absent in all non-epithelial cells.
Staining Pattern in Relevant Tumor Types
KRT14 expression is seen in the majority of squamous cell carcinomas irrespective of their origin and differentiation. KRT14 is also seen in almost all thymic epithelial tumors, in a large fraction of epitheloid type malignant mesothelioma and urothelial carcinomas, and – at lower frequency – also in other cancers.
The TCGA findings on Cytokeratin 14 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply MSVA-614R at a dilution of 1:50 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-614R 1:50 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-614R 1:50 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Roche – Ventana Discovery ULTRA
Pretreatment for 64 minutes at 100°C (pH 8,4); CM peroxidase blocking for 12 minutes (room temperature), MSVA-614R 1:50 for 20 minutes at 36°C, secondary antibody (anti-rabbit HQ) for 12 minutes at 36°C, anti-HQ HRP for 12 minutes at room temperature, DAB at room temperature, hematoxylin II at room temperature for 8 minutes, bluing reagent at room temperature for 4 minutes.
These images depict staining results obtained by the protocol described above. It is of note, that the Ventana machines generally require higher antibody concentrations than other commonly used autostainers because the antibodies are automatically diluted during the procedure. Various other protocols can result in an identical result as shown above. A longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of secondary antibody and or the anti-HQ HRP result in stronger staining, potentially at the cost of more background staining.
Impact of pH
The strongest KRT14 staining by MSVA-614R is obtained at a pH 9,0. However, pH 7,8 results in only a slight reduction of the staining intensity as compared to pH9. We thus consider pH7,8 as optimal for manual staining because of the better tissue preservation at pH7,8 than at pH 9,0.
Potential Research Applications
- The diagnostic utility of KRT14 expression analysis should be investigated in a large cohort of tumors from different entities.
- The prognostic role of KRT14 expression needs to be evaluated for various tumor types.
- Identification of basal and myoepithelial cell types in the prostate, breast and salivary glands (in multicolor immunofluorescence)
Evidence for Antibody Specificity in IHC
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also obtained with other antibodies for the same target (independent antibody strategy).
For the antibody MSVA-614R specificity is documented by the strong concordance of the immunostaining with RNA expression data derived fromthe Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project which are all summarized in the Human Protein Atlas (Tissue expression Cytokeratin 14)
These RNA data only highlight organs covered by squamous epithelium and salivary glands as KRT14 expressing tissues. Immunostaining by using MSVA-614R was almost exclusively detected in these organs. In addition, a positive immunostaining was found in basal cells of the prostate and the respiratory epithelium as well as in amnion and chorion cells of the placenta. These basal cells constitute small subsets of the total amount of cells in these organs and may have been largely underrepresented in RNA analyses.
True expression of KRT14 in basal cells and chorion cells is supported by findings obtained with the antibody HPA023040 in the protein atlas database.
Moreover, no staining was seen in tissues notorious for non-specific IHC background such as colonic mucosa.
A faint KRT14 staining in glomeruli may represent a (tolerable) cross-reactivity of the antibody, as RNA and protein data of the protein atlas do not show such a staining.