295,00 € – 995,00 €
Product details
Synonyms = Beta-Granin; CGA; CHGA; Chromogranin A Parathyroid Secretory Protein 1; ER-37; Pancreastatin; Parastatin; Parathyroid Secretory Protein 1; Pituitary Secretory Protein I; SP-I; Vasostatin I or II
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-380R
Positive control = Appendix: An at least weak to moderate staining must be seen in the axons and ganglion cells of the peripheral nerves while mucosal neuroendocrine cells exhibit a strong staining (neuroendocrine cells are not sufficient as positive controls because they express CGA so strongly, that even inefficient staining will detect its expression)
Negative control = Appendix: Epithelial and smooth muscle cells should be negative.
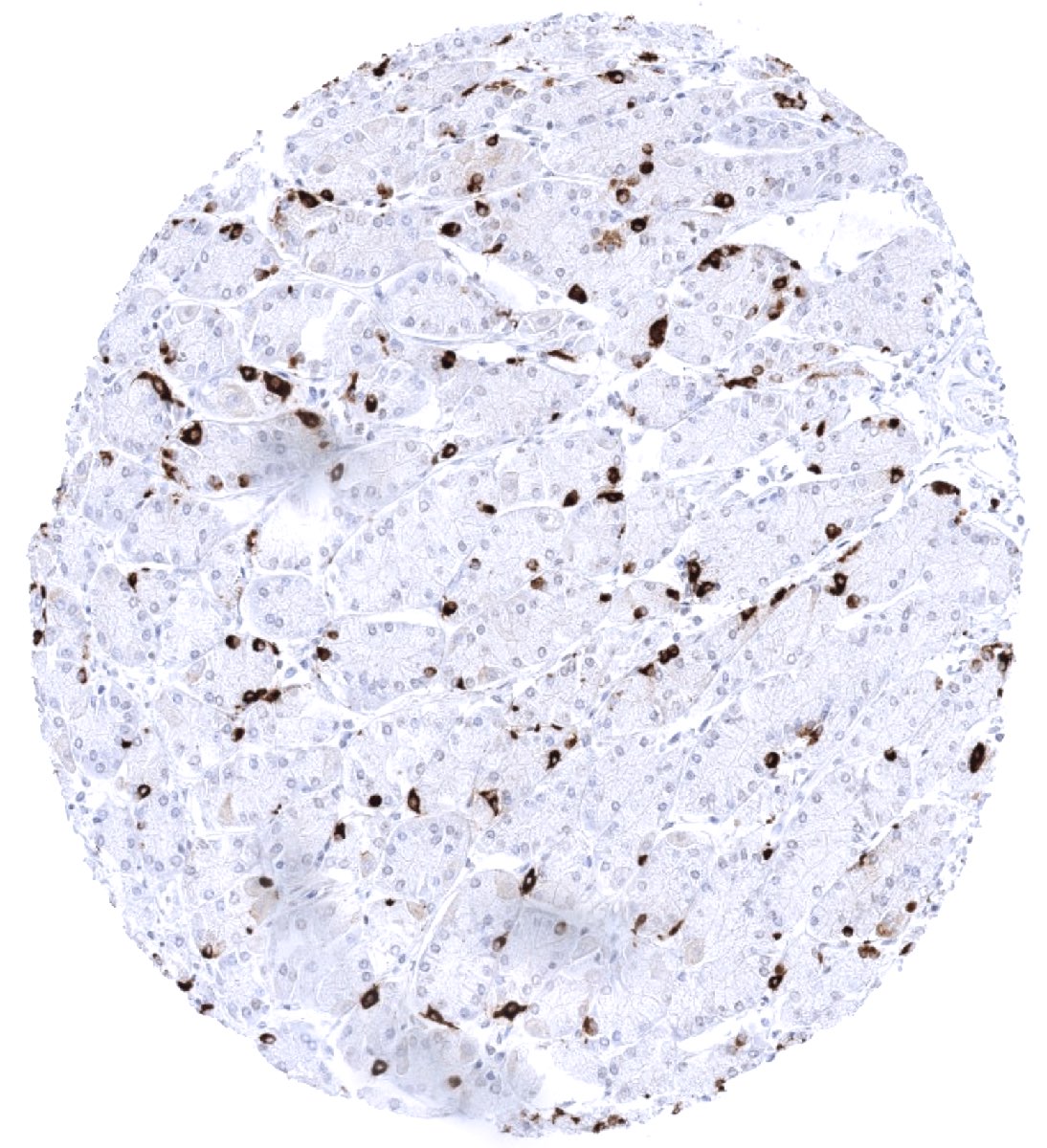
Cellular localization = Finely granular cytoplasmic
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
Chromogranin A is expressed on neuroendocrine cell types.
Biology Behind
Chromogranin A (CGA), a 48-75 kDa glycoprotein, is a member of the granin family of neuroendocrine secretory proteins. It is an important marker of the diffuse neuroendocrine system (DNES) and represents more than 50% of the soluble matrix protein in secretory granules of various types of neuroendocrine cells. Chromogranin A induces and promotes the generation of secretory vesicles of endocrine cells and neurons. Post-translational processing of the protein results in biologically active peptides that exert autocrine and paracrine functions. These peptides for example include chromostatin, chromacin, pancreastatin, catestatin, parastatin vasostatin-1, and vasostatin-2. The function of some of these peptides is known and affects mechanisms such as contraction and relaxation of vascular smooth muscle cells, microglial neurotoxin secretion, dopamine release from neural tissues or catecholamine release from the adrenal medulla.
Staining Pattern in Normal Tissues
Chromogranin A staining pattern in Normal Tissues with antibody MSVA-380R (images are shown in our “Normal Tissue Gallery”)
| Brain | Cerebrum | Negative. |
| Cerebellum | Negative. | |
| Endocrine Tissues | Thyroid | Negative. |
| Parathyroid | Strong chromogranin A positivity of all epithelial cells. | |
| Adrenal gland | Strong chromogranin A positivity in the medulla while adrenocortical cells are negative. | |
| Pituitary gland | Strong chromogranin A positivity of all epithelial cells of the adenohypophysis. | |
| Respiratory system | Respiratory epithelium | Negative. |
| Lung | Negative. | |
| Gastrointestinal Tract | Salivary glands | Negative. |
| Esophagus | Negative. | |
| Stomach | Strong chromogranin A staining of scattered neuroendocrine cells. Variable (mostly weak) chromogranin A staining of axons and ganglion cells of the peripheral nerves in the gastrointestinal wall. | |
| Duodenum | Strong chromogranin A staining of scattered neuroendocrine cells. Variable (mostly weak) chromogranin A staining of axons and ganglion cells of the peripheral nerves in the gastrointestinal wall. | |
| Small intestine | Strong chromogranin A staining of scattered neuroendocrine cells. Variable (mostly weak) chromogranin A staining of axons and ganglion cells of the peripheral nerves in the gastrointestinal wall. | |
| Appendix | Strong chromogranin A staining of scattered neuroendocrine cells. Variable (mostly weak) chromogranin A staining of axons and ganglion cells of the peripheral nerves in the gastrointestinal wall. | |
| Colon | Strong chromogranin A staining of scattered neuroendocrine cells. Variable (mostly weak) chromogranin A staining of axons and ganglion cells of the peripheral nerves in the gastrointestinal wall. | |
| Rectum | Strong chromogranin A staining of scattered neuroendocrine cells. Variable (mostly weak) chromogranin A staining of axons and ganglion cells of the peripheral nerves in the gastrointestinal wall. | |
| Liver | Negative. | |
| Gallbladder | Negative. | |
| Pancreas | Strong chromogranin A positivity of islet cells. | |
| Genitourinary | Kidney | Negative. |
| Urothelium | Negative. | |
| Male genital | Prostate | Negative. |
| Seminal vesicles | Negative. | |
| Testis | Negative. | |
| Epididymis | Negative. | |
| Female genital | Breast | Negative. |
| Uterus, myometrium | Negative. | |
| Uterus, ectocervix | Negative. | |
| Uterus endocervix | Negative. | |
| Uterus, endometrium | Negative. | |
| Fallopian Tube | Negative. | |
| Ovary | Negative. | |
| Placenta early | Negative. | |
| Placenta mature | Negative. | |
| Amnion | Negative. | |
| Chorion | Negative. | |
| Skin | Epidermis | Negative. |
| Sebaceous glands | Negative. | |
| Muscle/connective tissue | Heart muscle | Negative. |
| Skeletal muscle | Negative. | |
| Smooth muscle | Negative. | |
| Vessel walls | Negative. | |
| Fat | Negative. | |
| Stroma | Weak to moderate chromogranin A staining of axons and ganglion cells of peripheral nerves can be seen. | |
| Endothelium | Negative. | |
| Bone marrow/ lymphoid tissue | Bone marrow | Negative. |
| Lymph node | Negative. | |
| Spleen | Negative. | |
| Thymus | Negative. | |
| Tonsil | Negative. | |
| Remarks |
These findings are largely congruent to the data described in the Human Protein Atlas (Tissue expression Chromogranin A)
Suggested positive tissue control: Appendix: An at least weak to moderate staining must be seen in the axons and ganglion cells of the peripheral nerves while mucosal neuroendocrine cells exhibit a strong staining (neuroendocrine cells are not sufficient as positive controls because they express CGA so strongly, that even inefficient staining will detect its expression)
Suggested negative tissue control: Appendix: Epithelial and smooth muscle cells should be negative.
Staining Pattern in Relevant Tumor Types
Chromogranin is typically expressed at high levels in pheochromocytoma, paraganglioma, neuroendocrine tumors, neuroendocrine carcinomas, medullary carcinomas of the thyroid, pituitary adenomas, Merkel cell carcinomas and in small cell carcinomas irrespective of their site of origin. Chromogranin is also seen in various neuronal tumors such as neuroblastoma, ganglioneuroblastoma, ganglioneuroma, and ganglioglioma. Chromogranin may also be detected in brain tumors like oligodendroglioma, astrocytoma and ependymoma, however, to a varying extent. Moreover, chromogranin can be detected in a small fraction of various other tumor entities, such as for example breast, colorectal, prostate or ovarian cancers if these tumors exhibit neuroendocrine differentiation.
The TCGA findings on Chromogranin A RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
Chromogranin A (MSVA-380R) publication summary
Relevant publication: Uhlig et al. “Synaptophysin and chromogranin A expression analysis in human tumors.”. Published in Mol Cell Endocrinol. 2022 Sep 15;555:111726. PMID: 35921917.
A total of 11’218 tumors were successfully analyzed from 103 different tumor categories by using the following protocol: Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH7,8 Target Retrieval Solution buffer. MSVA-380R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent). This protocol was also used for all stainings depicted in our tumor and normal tissue galleries.
In this study, at least one positive case was seen in 51 (43.6%) of 117 tumor categories and 32 (27.4%) tumor categories included at least one case with strong positivity. The highest frequencies of chromogranin A positivity (and the highest levels of expression) were seen in various types of neuroendocrine neoplasms (52.2-100%), medullary thyroid carcinoma (100%), pheochromocytoma (97.9%), parathyroid gland adenoma (97.6%), paraganglioma (87.8%), small cell carcinoma of the lung (60%) basal cell carcinomas of the skin (56.3%), acinar cell carcinomas of the pancreas (40%), endometrioid endometrium carcinomas (36.5%), and in mucinous carcinomas of the ovary (35.8%). 7 additional entities showed positivity in 10-20% of cases, 10 further entities in 5-10% of cases, and 14 entities in <5% of cases. The distribution of positive staining results is shown in an “organ-systematic” and in a “ranking order” figure below (images based on data from Uhlig et al.). Results on possible associations with histopathological and clinical parameters of tumor aggressiveness are also summarized below (table based on data from Uhlig et al.).
Authors conclusions on diagnostic utility of chromogranin A immunohistochemistry with respect to the distinction of different tumor entities (Uhlig et al.):
- Distinction of neuroendocrine tumors or tumors with neuroendocrine differentiation from other tumors (as part of a panel).
- Distinction of pheochomocytoma (almost always positive) from adrenocortical tumors (almost always negative).
Authors conclusions on potential diagnostic pitfalls of chromogranin A immunohistochemistry (Uhlig et al.):
- Chromogranin A positivity can – in principle – occur in every tumor entity. Do not overinterpret expression of neuroendocrine markers in tumors lacking neuroendocrine-like morphology.
- Chromogranin A positive basal cell carcinoma of the skin must not be misinterpreted as Merkel cell carcinoma.
- Chromogranin A positive acinar cell carcinoma of the pancreas must not be misinterpreted as neuroendocrine tumor/carcinoma.
Authors conclusions on prognostic/predictive role of chromogranin A expression (Uhlig et al.):
- Chromogranin A positivity was largely unrelated to histopathological features of cancer aggressiveness in endometrium cancers, pancreatic adenocarcinomas, gastric adenocarcinomas and colorectal adenocarcinomas.
- Chromogranin A positivity was linked to ER loss in breast cancer (p=0,0213).
Because chromogranin A is typically used in combination with synaptophysin, data on x tumors that were successfully analyzed for both markers (Uhlig et al) are shown below in a combined ranking order. These combined set of data shows that both antibodies usually stain the same tumors but also highlights two major exceptions: Basal cell carcinomas of the skin are positive for chromogranin A but not for synaptophysin. Adrenocortical adenomas and carcinomas are often positive for synaptophysin but not for chromogranin A.
Figure 1. Chromogranin A staining in cancer (“organ-systematic”; according to Uhlig et al.)
Figure 2. Chromogranin A staining in cancer (“ranking list”; according to Uhlig et al.)
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
-Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply MSVA-380R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
-Impact of pH
For antibody MSVA-380R pH9 is optimal but pH7,8 results in only slightly weaker stainings. At pH6, Chromogranin A immunostaining is markedly reduced but still acceptable.
Potential Research Applications
- The prevalence and clinical significance of chromogranin A immunostaining in cancers of different types should be thoroughly evaluated.
- The role of neuroendocrine trans-differentiation in cancer is of critical interest, especially in prostate cancer.
Evidence for Antibody Specificity in IHC
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
For the antibody MSVA-380R specificity is documented by the strong concordance of the immunostaining with RNA expression data derived from the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project which are all summarized in the Human Protein Atlas (Tissue expression Chromogranin A) Immunostaining by using MSVA-380R was only detected in organs with documented RNA expression.
Moreover, no staining was seen in tissues notorious for non-specific IHC background such as kidney and the epidermis.