295,00 € – 995,00 €
Product details
Synonyms = STARD1
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-740R
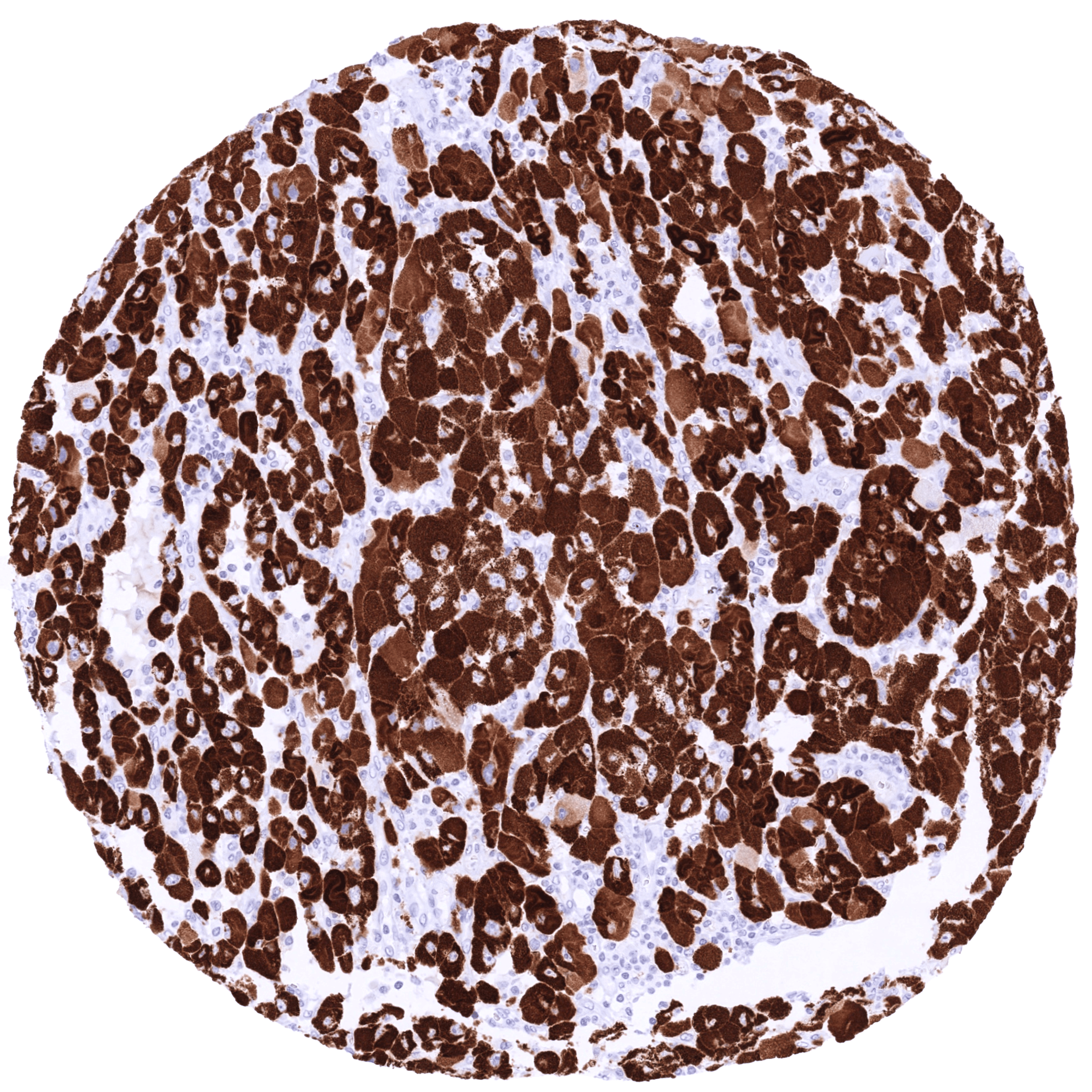
Positive control = Adrenal gland: A moderate to strong cytoplasmic StAR staining should be seen in the vast majority of adrenocortical cells.
Negative control = Colon: All cells must not show any StAR staining.
Cellular localization = Intracellular
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
StAR is a pivotal molecule for the synthesis of steroids.
Biology Behind
Steroidogenic acute regulatory (StAR) protein is a mitochondrial transport protein composed of 285 amino acids coded by the STARD1 gene at chromosome 8p11.2. StAR has a regulatory role for the transport of cholesterol from the outer mitochondrial membrane to the inner membrane which is a rate-limiting step for steroid hormone production. The mechanism by which StAR causes cholesterol movement is not fully understood but it has been suggested that StAR exerts its function from the outside of the mitochondria. StAR can be rapidly synthesized. Steroid synthesis is induced by hormones such as luteinizing hormone (LH), ACTH, and angiotensin II. Phosphorylation of the serine at position 195 increases StAR activity. Congenital StAR mutations cause lipoid congenital adrenal hyperplasia (lipoid CAH) which is characterized by in a severe shortage of steroid hormones after birth.
Staining Pattern in Normal Tissues
Images describing the StAR staining pattern in normal tissues obtained by the antibody MSVA-740R are shown in our “Normal Tissue Gallery”.
| Brain | Cerebrum | Negative. |
| Cerebellum | Negative. | |
| Endocrine Tissues | Thyroid | Negative. |
| Parathyroid | Negative. | |
| Adrenal gland | STAR staining of adrenocortical cells is usually strong but can be weaker focally or in individual samples. This variability may be due to the functional status of the cells. | |
| Pituitary gland | Negative. | |
| Respiratory system | Respiratory epithelium | Negative. |
| Lung | Negative. | |
| Gastrointestinal Tract | Salivary glands | Negative. |
| Esophagus | Negative. | |
| Stomach | Negative. | |
| Duodenum | Negative. | |
| Small intestine | Negative. | |
| Appendix | Negative. | |
| Colon | Negative. | |
| Rectum | Negative. | |
| Liver | Negative. | |
| Gallbladder | Negative. | |
| Pancreas | Negative. | |
| Genitourinary | Kidney | Negative. |
| Urothelium | Negative. | |
| Male genital | Prostate | Negative. |
| Seminal vesicles | Negative. | |
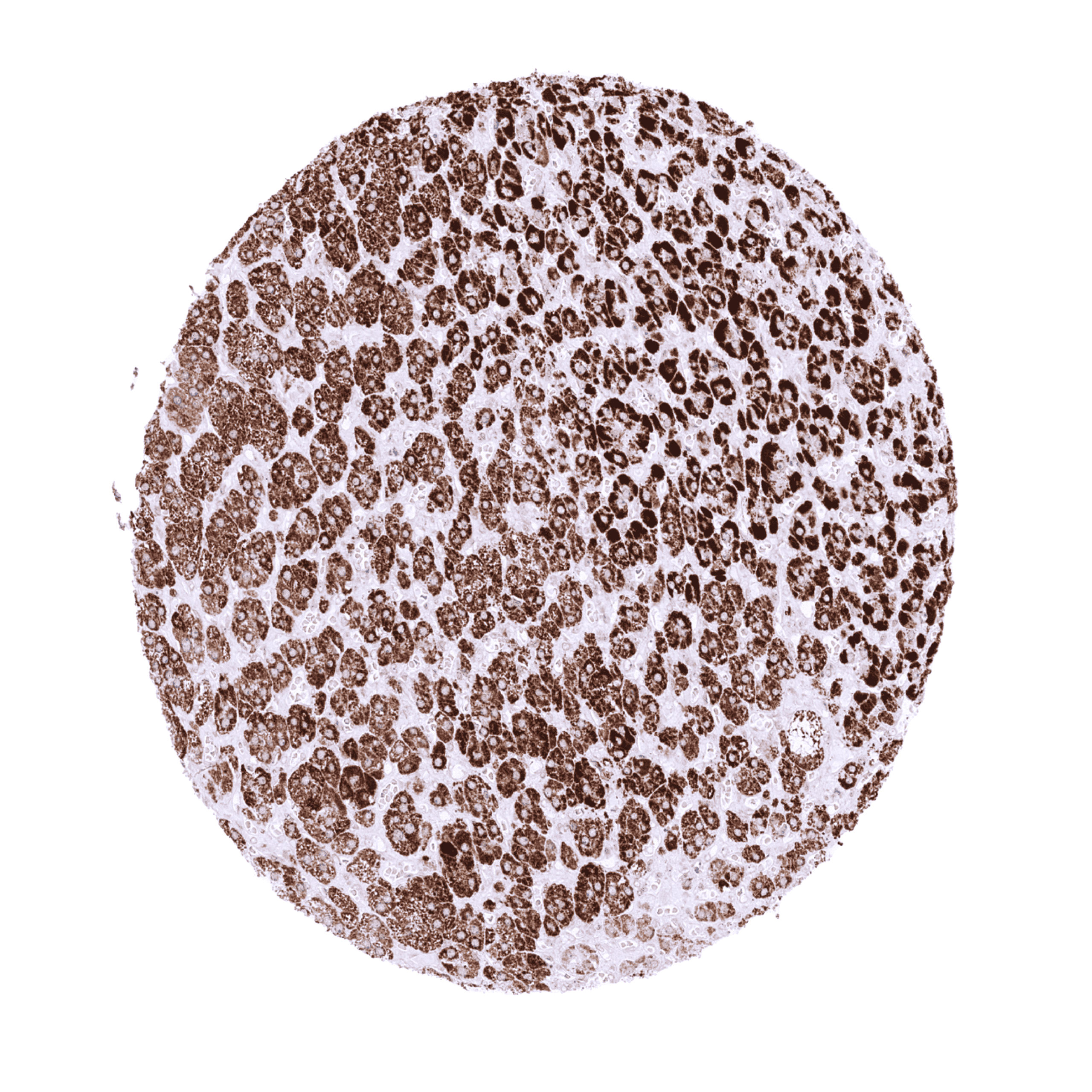
| Testis | Strong STAR staining in Leydig cells. | |
| Epididymis | Negative. | |
| Female genital | Breast | Negative. |
| Uterus, myometrium | Negative. | |
| Uterus, ectocervix | Negative. | |
| Uterus endocervix | Negative. | |
| Uterus, endometrium | Negative. | |
| Fallopian Tube | Negative. | |
| Ovary | Strong cytoplasmic staining in Corpus luteum cells and theca interna cells of follicular cysts. A weaker staining can be seen in granulosa cells (not in all samples). | |
| Placenta early | Negative. | |
| Placenta mature | Negative. | |
| Amnion | Negative. | |
| Chorion | Negative. | |
| Skin | Epidermis | Negative. |
| Sebaceous glands | Negative. | |
| Muscle/connective tissue | Heart muscle | Negative. |
| Skeletal muscle | Negative. | |
| Smooth muscle | Negative. | |
| Vessel walls | Negative. | |
| Fat | Negative. | |
| Stroma | Negative. | |
| Endothelium | Negative. | |
| Bone marrow/ lymphoid tissue | Bone marrow | Negative. |
| Lymph node | Negative. | |
| Spleen | Negative. | |
| Thymus | Negative. | |
| Tonsil | Negative. | |
| Remarks |
Positive control = Adrenal gland: A moderate to strong cytoplasmic StAR staining should be seen in the vast majority of adrenocortical cells.
Negative control = Colon: All cells must not show any StAR staining.
Staining Pattern in Relevant Tumor Types
The TCGA findings on STAR RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply MSVA-740R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Potential Research Applications
- The expression of StAR in different tumor entities needs to be investigated.
- Recent studies suggested, that StAR can be expressed in several tissues that produce steroid hormones for local use, potentially conferring some functional advantage by intracrine, autocrine or paracrine effects. This mechanism needs to be further investigated.
- The mechanism by which StAR exerts its function is unclear.
Evidence for Antibody Specificity in IHC
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. Comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
Orthogonal validation: For the antibody MSVA-740R specificity is suggested by the strong concordance of the immunostaining data with data from three independent RNA screening studies, including the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project, which are all summarized in the Human Protein Atlas (Tissue expression STAR). RNA expression was only described in these organs where a positive StAR immunostaining was seen by MSVA-740R(adrenal gland, ovary, testis).
Comparison of antibodies: True expression of StAR in the cell types identified by MSVA-740R (Leydig cells of the testis, adrenocortical cells, granulosa and theca interna cells of the ovary) was confirmed by comparison with a commercially available independent second antibody (termed “validation antibody”).