Product details
Synonyms = 5′-nucleotidase (5′-NT); Acid phosphatase prostate; ACP3; Ecto-5′-nucleotidase; Prostatic acid phosphatase (PAP); Prostatic acid phosphatase; Thiamine monophosphatase (TMPase)
Antibody type = Recombinant Mouse monoclonal / IgG1
Clone = MSVA-452M
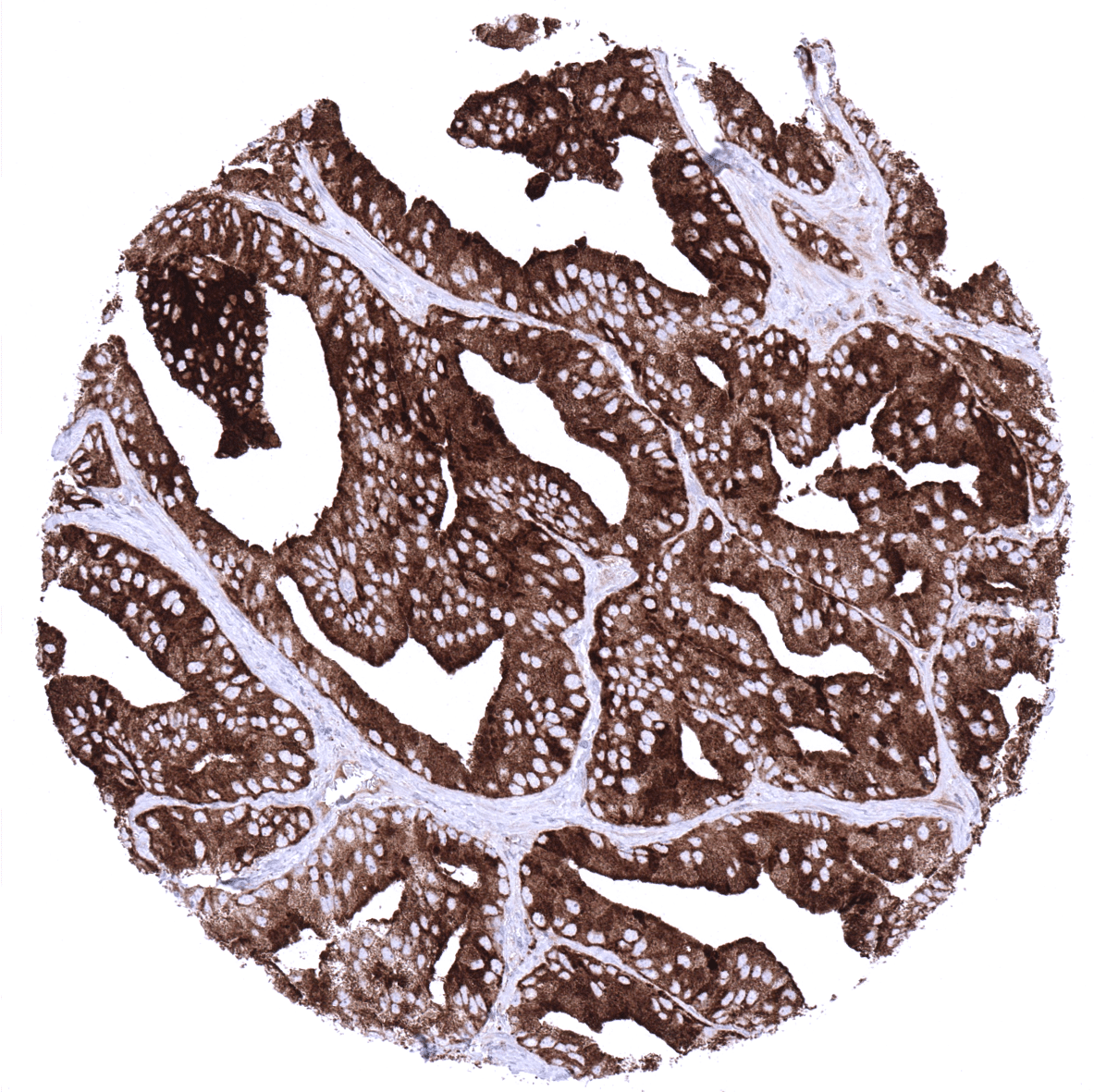
Positive control = Prostate: A strong PSAP immunostaining should, be seen in all glandular cells.
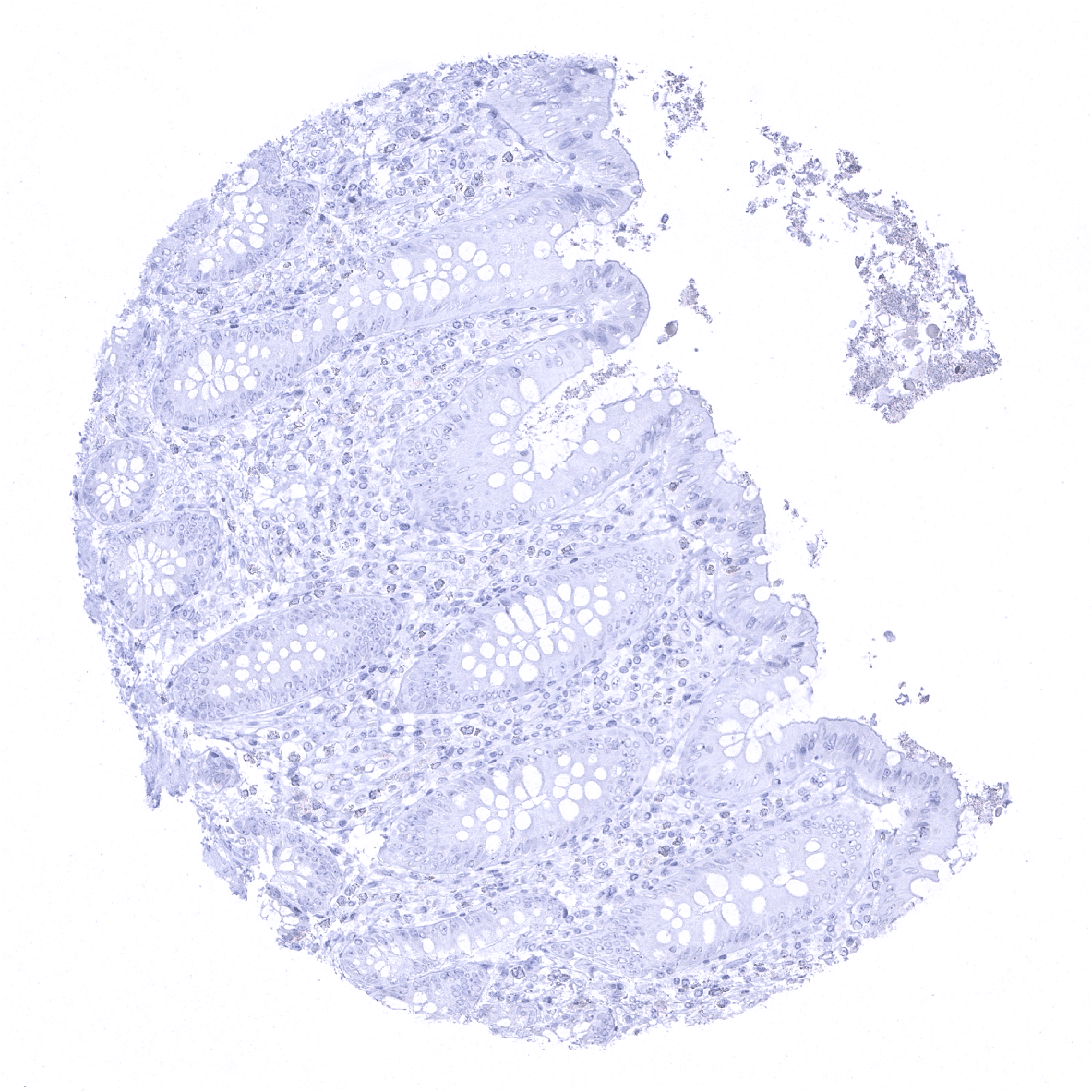
Negative control = Appendix: All epithelial and non-epithelial structures must not show any PSAP immunostaining.
Cellular localization = Cytoplasmic
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
PSAP is expressed in normal and neoplastic acinar cells of the prostate.
Biology Behind
Prostatic specific acid phosphatase (PSAP), also termed Prostatic acid phosphatase (PAP) is coded by a gene at 3q21-23. It is an enzyme that is produced in prostate epithelial cells and has the capacity to dephosphorylate macromolecules in acidic conditions (pH 4-6). Its substrates are not fully known. It is suspected, that PSAP’s physiological function may be related to the liquefaction process of semen. Alternative splicing generates two types of PAP transcripts, a transmembrane PAP consisting of 11 exons, and cellular and a secretory PAPs containing 10. The molecular mechanisms controling PSAP protein expression are not fully understood. Factors involved in the regulation of PSAP expression include androgen, androgen receptor, NF-κB, TNF-a and IL-1.
Staining Pattern in Normal Tissues
In normal tissues, PSAP is found in prostate glandular cells and at a lower level in sebaceous glands. PSAP immunostaining is absent in all other tissues including all epithelial cells of the gastrointestinal and the genitourinary tract, fallopian tubes, endometrium, endocervix, placenta, gallbladder, liver, pancreas, salivary and bronchial glands, breast gland, Brunner glands, thyroid, pituitary gland, adrenal gland, parathyroid gland, testis, epididymis, seminal vesicle, non-keratinizing and keratinizing squamous epithelium of various different sites, skin appendices, all mesenchymal tissues, hematopoetic and immune cells, and the brain.
These findings are largely comparable to the RNA and protein data described in the Human Protein Atlas (Tissue expression PSAP)
Suggested positive tissue control: Prostate: A strong PSAP immunostaining should, be seen in all glandular cells.
Suggested negative tissue control: Appendix: All epithelial and non-epithelial structures must not show any PSAP immunostaining.
Staining Pattern in Relevant Tumor Types
PSAP is expressed in the vast majority of adenocarcinomas of the prostate. The prevalence of positive cases was described to decrease slightly with increasing Gleason score. PSAP is also described to be positive in tumors derived from the Skene’s glands, and in a fraction of salivary gland tumors. PSAP positivity was also described to potentially occur in nephrogenic adenoma, as well as male and female breast cancers.
The TCGA findings on PSAP RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein. Accordingly, multiple different protocols can generate identical staining results.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH9 Target Retrieval Solution buffer. Apply MSVA-452M at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-452M 1:150 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-452M 1:150 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Roche – Ventana Discovery ULTRA
Pretreatment for 64 minutes at 100°C (pH 8,4); CM peroxidase blocking for 12 minutes (room temperature), MSVA-452M 1:150 for 20 minutes at 36°C, secondary antibody (anti-mouse HQ) for 12 minutes at 36°C, anti-HQ HRP for 12 minutes at room temperature, DAB at room temperature, hematoxylin II at room temperature for 8 minutes, bluing reagent at room temperature for 4 minutes.
These images depict staining results obtained by the protocol described above. It is of note, that the Ventana machines generally require higher antibody concentrations than other commonly used autostainers because the antibodies are automatically diluted during the procedure. Various other protocols can result in an identical result as shown above. A longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of secondary antibody and or the anti-HQ HRP result in stronger staining, potentially at the cost of more background staining.
Potential Research Applications
- The diagnostic utility of PSAP expression analysis should be investigated in a large cohort of tumors from different entities.
- The prognostic role of PSAP expression levels in tissue and serum are not entirely clear.
Evidence for Antibody Specificity in IHC
Specificity of MSVA-452M is documented by strong positive staining in cell types that are well documented to express PSAP such as prostate epithelium and of sebaceous glands and absence of staining in all other tissues including those being notorious for non-specific IHC background such as kidney, colonic mucosa, and epidermis.