295,00 € – 995,00 €
Product details
Synonyms = Alkaline phosphatase placental type; Alkaline phosphatase Regan Isozyme; ALP; Alp1; ALPP; Germ-cell alkaline phosphatase; nagao Isozyme; PALP; Placental alkaline phosphatase 1; placental heat-stable alkaline phosphatase; PLAP-1; PLAP1
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-350R
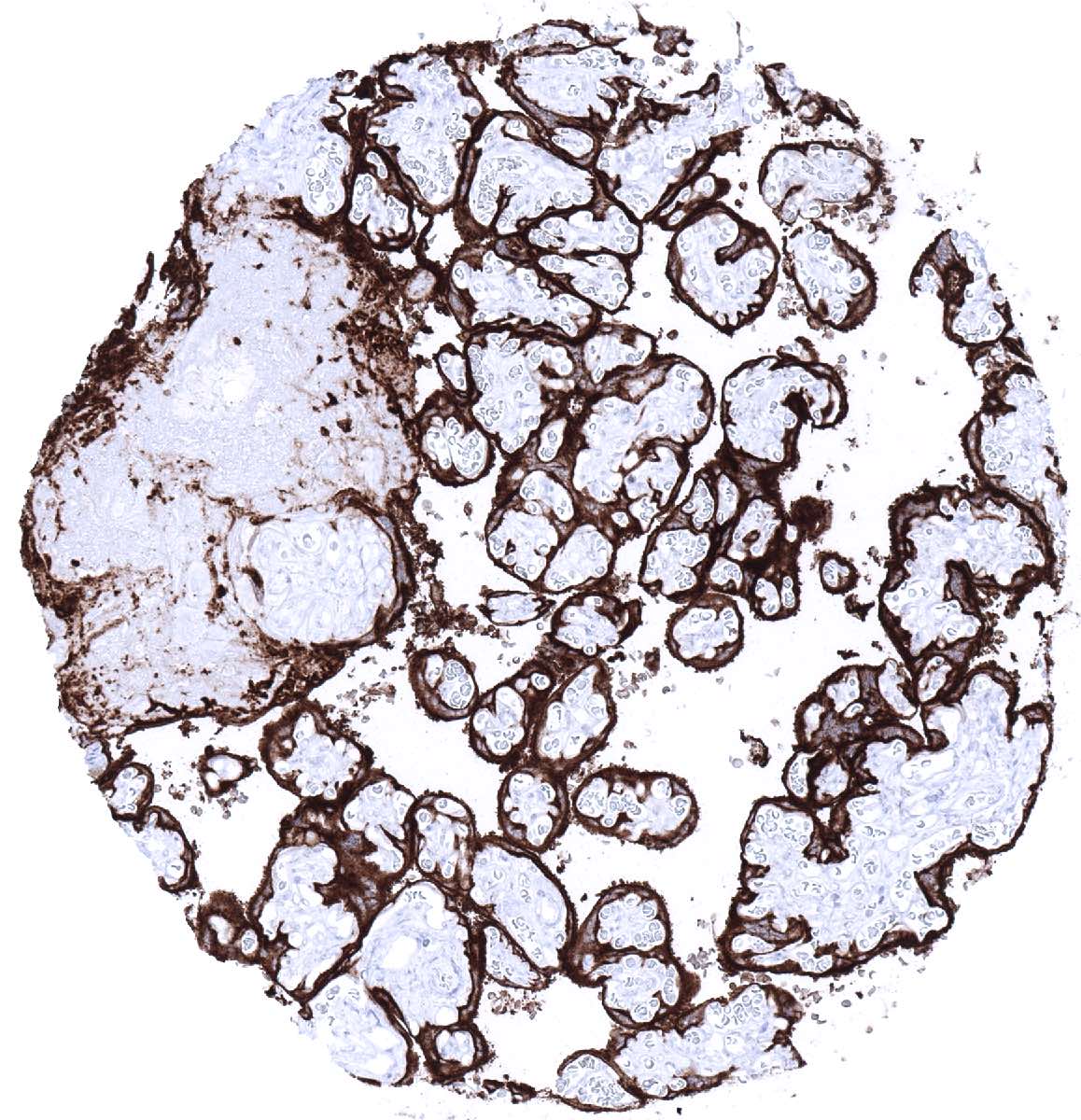
Positive control = Placenta (mature): A strong membranous and cytoplasmic staining of cytotrophoblast and syncytiotrophoblast must be seen.
Negative control = Appendix: PLAP immunostaining must be absent in all structures.
Cellular localization = Cell Surface and Cytoplasmic
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
PLAP is a marker for germ cell tumors which can also be expressed in various types of adenocarcinomas.
Biology Behind
Placental alkaline phosphatase (PLAP) is a 58 KDa enzyme encoded by the ALPP gene at the human chromosome 2q37.1. The highly polymorphic ALPP gene is expressed in 18 different allelic variants which, however, all share the same function. The transition from the pre-mature to the mature form of the enzyme involves cleavage of the pro-peptide portion spanning amino acids 507-535. Mature PLAP is bound to the plasma membrane. Homodimerization of two PLAP molecules is required to form the catalytic center. The physiological role of PLAP is still unknow, but it might aid in guiding migratory cells and transport specific molecules through the plasma membrane. PLAP becomes expressed in the maturing placenta with steadily raising levels during pregnancy. Three of the different PLAP isoforms have been attributed to to early, mid-term and term placenta.[1]
[1] Reiswich et al Reiswich et al “Pattern of placental alkaline phosphatase (PLAP) expression in human tumors: a tissue microarray study on 12,381 tumors” The journal of Pathology: Clinical Research, August 7th 2021
Staining Pattern in Normal Tissues
PLAP staining pattern in Normal Tissues with antibody MSVA-350R (images are shown in our “Normal Tissue Gallery”)
| Brain | Cerebrum | Negative. |
| Cerebellum | Negative. | |
| Endocrine Tissues | Thyroid | Negative. |
| Parathyroid | Negative. | |
| Adrenal gland | Negative. | |
| Pituitary gland | Negative. | |
| Respiratory system | Respiratory epithelium | Negative. |
| Lung | Focal membranous PLAP staining of apical membranes of pneumocytes. | |
| Gastrointestinal Tract | Salivary glands | Negative. |
| Esophagus | Negative. | |
| Stomach | Negative. | |
| Colon | Negative. | |
| Duodenum | Negative. | |
| Rectum | Negative. | |
| Small intestine | Negative. | |
| Liver | Negative. | |
| Gallbladder | Negative. | |
| Pancreas | Negative. | |
| Genitourinary | Kidney | Negative. |
| Urothelium | Negative. | |
| Male genital | Prostate | Negative. |
| Seminal vesicles | Negative. | |
| Testis | Negative. | |
| Epididymis | Negative. | |
| Female genital | Breast | Negative. |
| Uterus, ectocervix | Negative. | |
| Uterus endocervix | A weak PLAP staining can be seen at the surface apical membrane of epithelial cells (not in all samples). | |
| Uterus, endometrium | A weak PLAP staining can be seen at the surface apical membrane of epithelial cells (not in all samples). | |
| Fallopian Tube | A weak PLAP staining can be seen at the surface apical membrane of epithelial cells (not in all samples). | |
| Ovary | Negative. | |
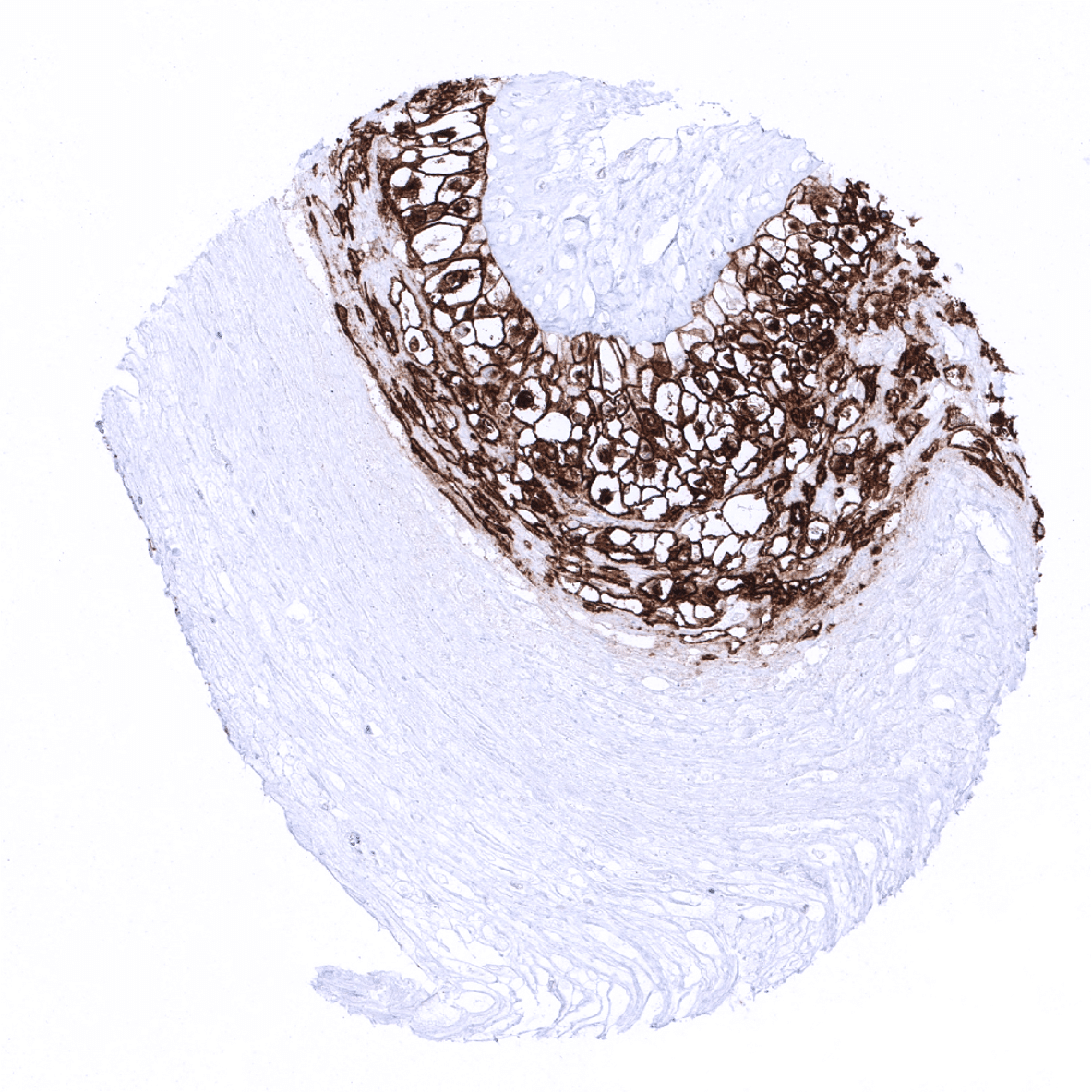
| Placenta early | PLAP staining is only moderate and limited to the surface cell membrane of trophoblast cells. | |
| Placenta mature | Strong PLAP staining of the cytotrophoblast and the syncytiotrophoblast. | |
| Amnion | Weak PLAP staining of amnion cells. | |
| Chorion | Strong PLAP staining of chorion cells. | |
| Skin | Epidermis | Negative. |
| Sebaceous glands | Negative. | |
| Muscle/connective tissue | Heart | Negative. |
| Skeletal | Negative. | |
| Smooth muscle | Negative. | |
| Fat | Negative. | |
| Bone marrow/lymphoid | Bone marrow | Negative. |
| Lymph node | Negative. | |
| Spleen | Negative. | |
| Thymus | Negative. | |
| Tonsil | Negative. | |
| Remarks |
These findings are highly consistent with the RNA and protein data described in the Human Protein Atlas (Tissue expression PLAP). The protein atlas data also suggest the uterus as an alternative source of limited PLAP expression besides placenta. That lung was also described in the protein atlas as a source of very limited PLAP RNA amounts cannot be explained by findings in this analysis.
Suggested positive tissue control: Placenta (mature): A strong membranous and cytoplasmic staining of cytotrophoblast, syncytiotrophoblast, and chorion cells must be seen.
Suggested negative tissue control: Appendix: PLAP immunostaining must be absent in all structures.
Staining Pattern in Relevant Tumor Types
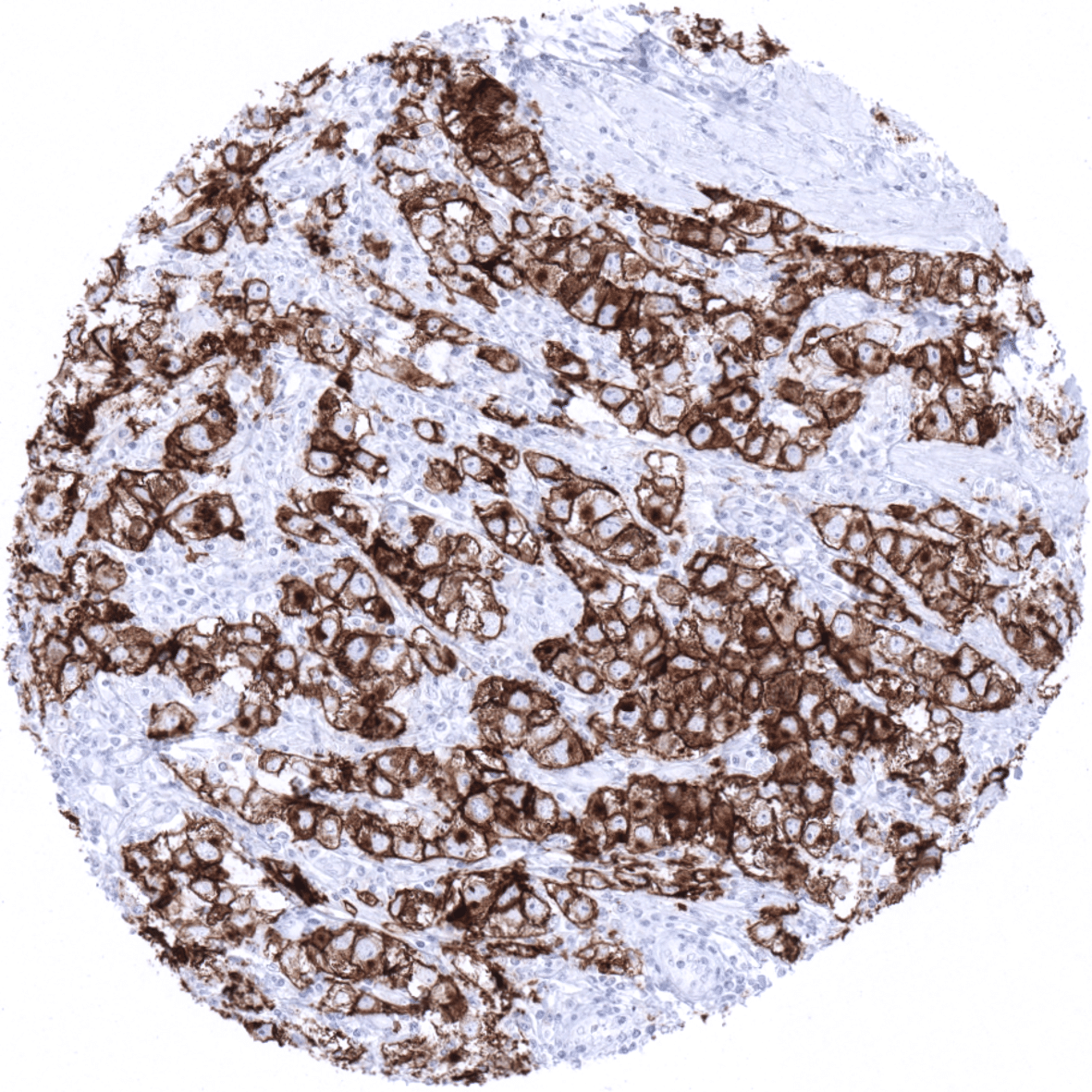
PLAP is consistently expressed at high levels in seminoma, dysgerminoma, germinoma, intratubular germ cell neoplasia, and hydatidiform moles. PLAP is – usually at lower levels – also often positive in embryonal carcinoma, yolk sack carcinomas, and choriocarcinoma. PLAP is usually absent in spermatocytic seminoma.
Detailed data on PLAP staining by MSVA-350R obtained from an analysis of 12,381 tumors from 131 different tumor types and subtypes have recently been published by Reiswich et al “Pattern of placental alkaline phosphatase (PLAP) expression in human tumors: a tissue microarray study on 12,381 tumors“
The authors found a moderate to strong PLAP positivity in seminoma (96%), embryonal carcinoma (85%), yolk sac tumors of the testis (56%), endometrioid carcinoma of the endometrium (28%) and the ovary (20%), gastric adenocarcinoma (22%), serous carcinoma of the ovary (17%) and the uterus (11%), adenocarcinoma of the ampulla of Vater (15%), carcinosarcoma of the ovary (11%) and the uterus (8%), esophageal adenocarcinoma (10%), invasive urothelial carcinoma (4%), cholangiocarcinoma (2%), and adenocarcinoma of the lung (1%). Low-level PLAP immunostaining, often involving only a small fraction of tumor cells, was seen in 21 additional tumor entities.
The TCGA findings on PLAP RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
PLAP (MSVA-350R) publication summary:
Relevant publication: Reiswich et al. “Pattern of placental alkaline phosphatase (PLAP) expression in human tumors: a tissue microarray study on 12,381 tumors“. Published in the journal of Pathology: Clinical Research, August 7th 2021
A total of 12,381 tumors were analyzed from 131 different tumor categories by using the following protocol: Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH9 Target Retrieval Solution buffer. MSVA-350R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent). This protocol was also used for all stainings depicted in our tumor and normal tissue galleries.
At least one case with a positive PLAP immunostaining was seen in 48 (36.6%) and at least one case with a strong PLAP immunostaining was seen in 22 (16.8%) of 131 tumor categories. The distribution of positive staining results is shown “organ-systematic” and in a “ranking order” figure below (images based on data from Reiswich et al). Results on possible associations with histopathological and clinical parameters of tumor aggressiveness are also summarized below (table based on data from Reiswich et al).
Authors conclusions on diagnostic utility with respect to the distinction of benign versus malignant (Reiswich et al):
- PLAP expression was not seen in normal testis but in a large fraction of germinal cell neoplasms.
Authors conclusions on diagnostic utility with respect to the distinction of different tumor entities (Reiswich et al):
- Besides germ cell tumors, which show the highest PLAP expression prevalence, high-level PLAP expression can be found in cancers from the female genital tract, the gastro- esophageal, and the pancreaticobiliary system as well as in a few other tumor types.
- Low-level PLAP expression can be found in various other tumor entities and should generally not be viewed as a strong argument for a germ cell neoplasm.
- PLAP expression does not occur in leiomyomatous tumors (previous reports on this subject were driven by cross-reactivity of other PLAP antibodies).
Authors conclusions on prognostic/predictive role of PLAP expression.
- Neo-expression (upregulation) of PLAP was linked to unfavorable tumor features in colorectal cancer.
- Low expression (downregulation) of PLAP was linked to advanced pT category in endometrial carcinoma.
Data from the publication: “Pattern of placental alkaline phosphatase (PLAP) expression in human tumors: a tissue microarray study on 12,381 tumors“. Published by Reiswich et al. in the journal of Pathology: Clinical Research, August 7th 2021.
Summarized in own graphics:
1. PLAP staining in tumors “organ-specific” with antibody MSVA-350R
2. PLAP staining in tumors “ranking order” by positivity with antibody MSVA-350R.
3. Table Clinico-pathological associations described by Reiswich et al. (p-value)
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein. Accordingly, multiple different protocols can generate identical staining results.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH9 Target Retrieval Solution buffer. Apply MSVA-350R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-350R 1:150 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-350R 1:150 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Roche – Ventana Discovery ULTRA
Pretreatment for 64 minutes at 100°C (pH 8,4); CM peroxidase blocking for 12 minutes (room temperature), MSVA-350R 1:150 for 20 minutes at 36°C, secondary antibody (anti-rabbit HQ) for 12 minutes at 36°C, anti-HQ HRP for 12 minutes at room temperature, DAB at room temperature, hematoxylin II at room temperature for 8 minutes, bluing reagent at room temperature for 4 minutes.
These images depict staining results obtained by the protocol described above. It is of note, that the Ventana machines generally require higher antibody concentrations than other commonly used autostainers because the antibodies are automatically diluted during the procedure. Various other protocols can result in an identical result as shown above. A longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of secondary antibody and or the anti-HQ HRP result in stronger staining, potentially at the cost of more background staining.
Impact of pH
The strongest PLAP staining by MSVA-350R is obtained at a pH 9,0. However, pH 7,8 results in only a slight reduction of the staining intensity as compared to pH9. We thus consider pH7,8 as optimal for manual staining because of the better tissue preservation at pH7,8 than at pH 9,0.
Potential Research Applications
- The clinical significance of PLAP expression in non-germ cell neoplasms and the function of PLAP in these tumors needs to be investigated.
Evidence for Antibody Specificity in IHC
Specificity of MSVA-350R is documented by strong positive staining in cell types that are documented to express PLAP such as placenta tissue and to a much lesser extent cervical/endometrium/fallopian tube and absence of staining in all other tissues including tissues notorious for non-specific IHC background such as kidney, colonic mucosa, and epidermis.
In a recent publication, the specificity of MSVA-350R was demonstrated in a comparison vs. another independent antibody. All normal tissues structures stained by MSVA-350R were also detected with the second independent antibody by Reiswich et al “Pattern of placental alkaline phosphatase (PLAP) expression in human tumors: a tissue microarray study on 12,381 tumors“.