Product details
Synonyms = Gracile Axonal Dystrophy; Neuron Cytoplasmic Protein 9.5; Park5; Parkinson Disease 5; PGP95; Protein Gene Product 9.5; Ubiquitin Carboxyl-terminal Esterase L1; Ubiquitin Carboxyl- terminal Hydrolase Isozyme L1; Ubiquitin Thioesterase L1; Ubiquitin Thiolesterase L1
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-905R
Positive control = Pancreas: Islets cells and interspersed nerve fibres should show strong PGP9.5 staining.
Negative control = Pancreas: Acinar cells and excretory ducts should not show any PGP9.5 immunostaining.
Cellular localization = Cytoplasmic. Endoplasmic Reticulum membrane.
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
PGP9.5 is expressed in neuronal cells and key component of the ubiquitination/deubiquitination system.
Biology Behind
PGP9.5, also termed Ubiquitin C-terminal hydrolase (UCH)-L1 belongs to the Ub C- terminal hydrolases (UCHs) representing one of five sub-families of de-ubiquitination enzymes (DUBs). PGP9.5/UCH-L1 is thus an important component of the ubiquitination/deubiquitination system and plays a role in the posttranslational modification of proteins including protein degradation, relocation and functional alteration. PGP9.5 is one of the most prevalent proteins in the brain and makes up for 1-2% of the brain protein mass. It occurs in the cytoplasm and axons of neurons. As part of the axonal skeleton, it plays a role in axonal transport. Elevated serum PGP9.5 is a valuable marker for traumatic brain injury, and PGP9.5 serum levels correlate with the severity of injury and clinical outcome. Altered function and expression of UCH-L1 has been linked to neurodegenerative disease, cancer and other diseases. Several mutations and functional alterations of PGP9.5 have been found to be linked to neurodegenerative disease. Moreover, overexpression of PGP9.5 has been found in several cancer types, including pancreatic cancer, myeloma, prostate cancer, neuroblastoma, and osteosarcoma. Altered PGP9.5 expression in cancer has been found to be correlated with cancer cell chemotherapy resistance, metastasis, and patient prognosis.
Staining Pattern in Normal Tissues
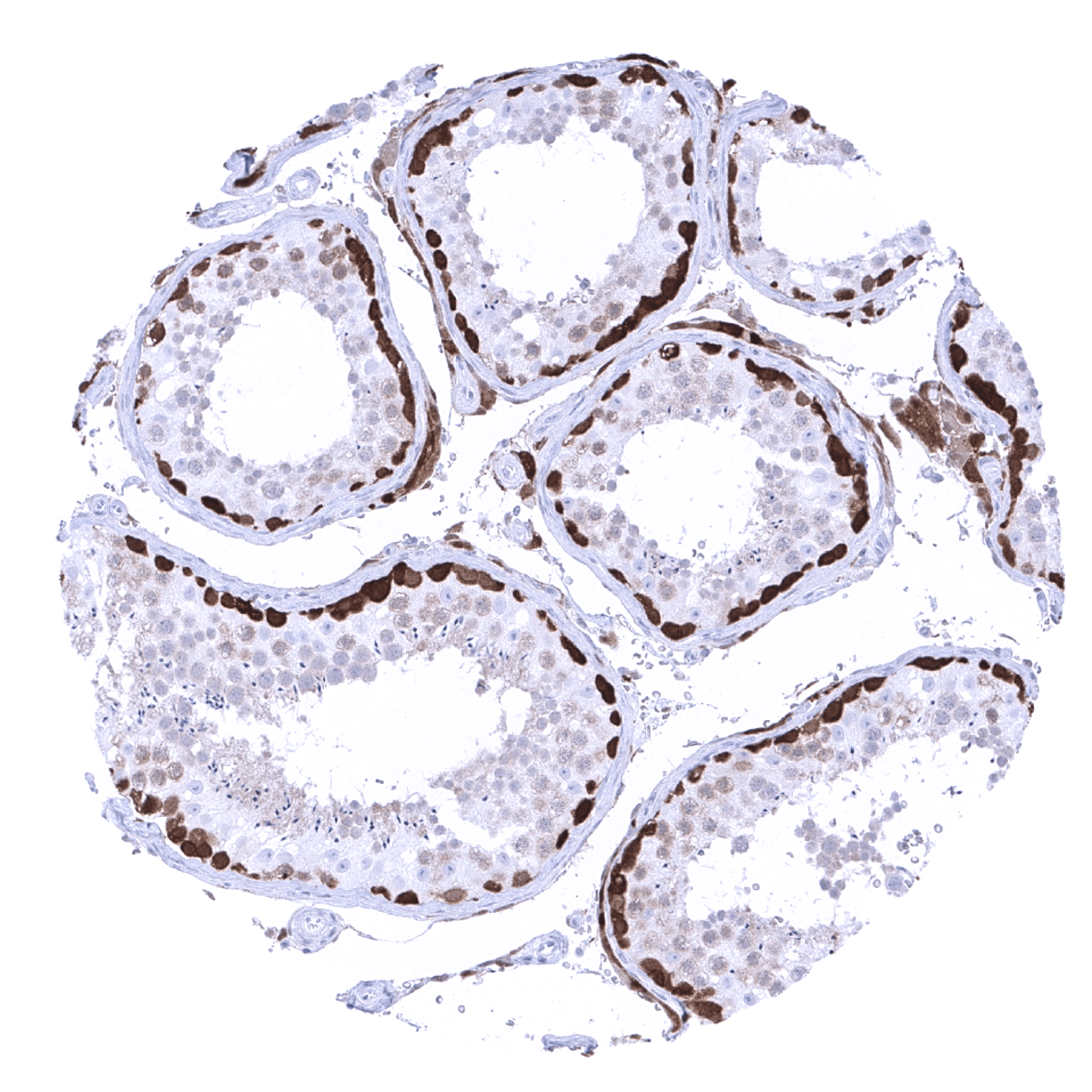
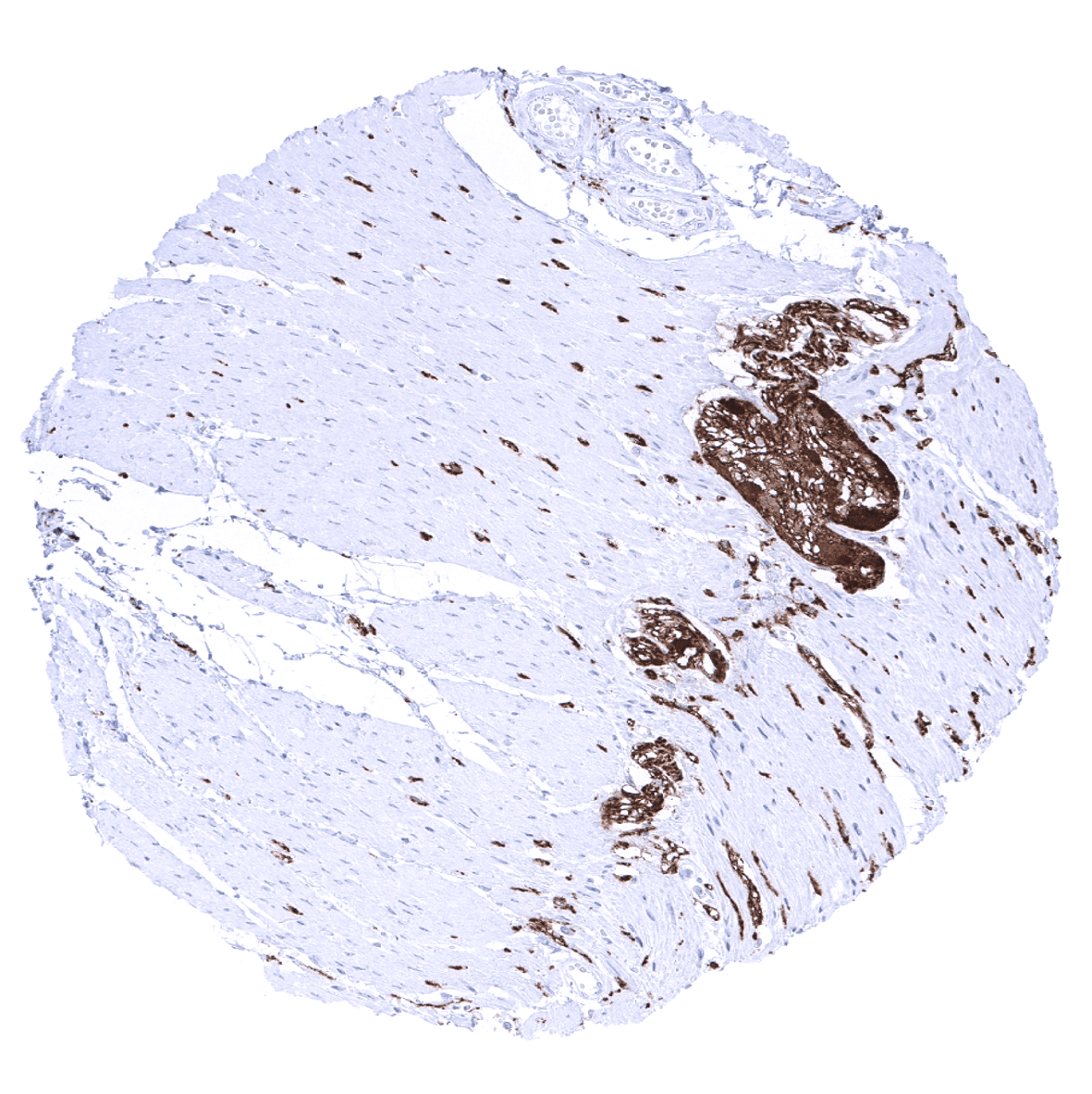
The strongest PGP9.5 immunostaining is seen in all neuronal cells (brain and ganglia) and corresponding axons in the brain, neurohypophysis and in peripheral nerves. A similarly strong staining occurs in spermatogonia of the testis, the adrenal medulla, islet cells of the pancreas, and in corpora lutea of the ovary. A moderate to strong PGP9.5 positivity is also seen in Leydig cells, the theca cell layer of follicular cysts, ovarian stroma, the adenohypophysis, and in some scattered (neuroendocrine?) cells in the thymus. In several epithelial tissues, scattered PGP9.5 positive cells or groups of cells which often show a mosaic pattern with high variability of the expression levels between neighboring cells can occur. These include respiratory epithelium, fallopian tube, cauda epididymis, and (rarely) the prostate. A weak PGP9.5 staining occurs in a fraction of lymphocytes, predominantly located in the germinal centres. Decidua cells show a variable level of PGP9.5 expression.
These findings are largely comparable to the RNA and protein data obtained by the antibody (HPA005993) described in the protein atlas.
Positive control: Pancreas: Islets cells and interspersed nerve fibres should show strong PGP9.5 staining.
Negative control: Pancreas: Acinar cells and excretory ducts should not show any PGP9.5 immunostaining.
Staining Pattern in Relevant Tumor Types
High level PGP9.5 expression is common in all kinds of neural and neuroendocrine tumors but can also occur at variable frequency in a large number of additional tumor types.
The TCGA findings on PGP9.5 / UCHL1 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply MSVA-905R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-905R 1:150 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-905R 1:200 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Roche – Ventana Discovery ULTRA
Pretreatment for 64 minutes at 100°C (pH 8,4); CM peroxidase blocking for 12 minutes (room temperature), MSVA-905R 1:50 for 20 minutes at 36°C, secondary antibody (anti-rabbit HQ) for 12 minutes at 36°C, anti-HQ HRP for 12 minutes at room temperature, DAB at room temperature, hematoxylin II at room temperature for 8 minutes, bluing reagent at room temperature for 4 minutes.
These images depict staining results obtained by the protocol described above. It is of note, that the Ventana machines generally require higher antibody concentrations than other commonly used autostainers because the antibodies are automatically diluted during the procedure. Various other protocols can result in an identical result as shown above. A longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of secondary antibody and or the anti-HQ HRP result in stronger staining, potentially at the cost of more background staining.
Potential Research Applications
- The clinical/prognostic role of PGP9.5 expression needs to be evaluated in cancer.
- Visualization and quantification of nerve fibres and ganglions in the colon.
- Visualization and quantification of undifferentiated spermatogonia in the testis.
Evidence for Antibody Specificity in IHC
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
Orthogonal validation: For the antibody MSVA-905R specificity is suggested by the strong concordance of the immunostaining data with data from three independent RNA screening studies, including the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project, which are all summarized in the Human Protein Atlas (Tissue expression PGP9.5)
Immunostaining by using MSVA-905R was almost exclusively detected in organs with documented PGP9.5 RNA expression such as the brain, adrenal gland, pituitary gland, kidney, and the ovary. In addition, a positive immunostaining was also found in testis, epididymis, bronchus mucosa, fallopian tube, and the pancreas. In these organs, the PGP9.5 positive cell types constituted rather small subsets of the total amount of cells and may therefore have been largely underrepresented in RNA analyses.
Comparison of antibodies: True expression of PGP9.5 in these organs is suggested by the results described for the independent anti-PGP9.5 antibodies HPA005993 and CAB002580 in the protein atlas. Images showing comparable results are shown for:
Moreover, no staining was seen in tissues notorious for non-specific IHC background such as colonic mucosa, and epidermis.