295,00 € – 995,00 €
Product details
Synonyms = A1; Insulinoma associated protein 1 (IA1); Zinc finger protein IA1
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-456R
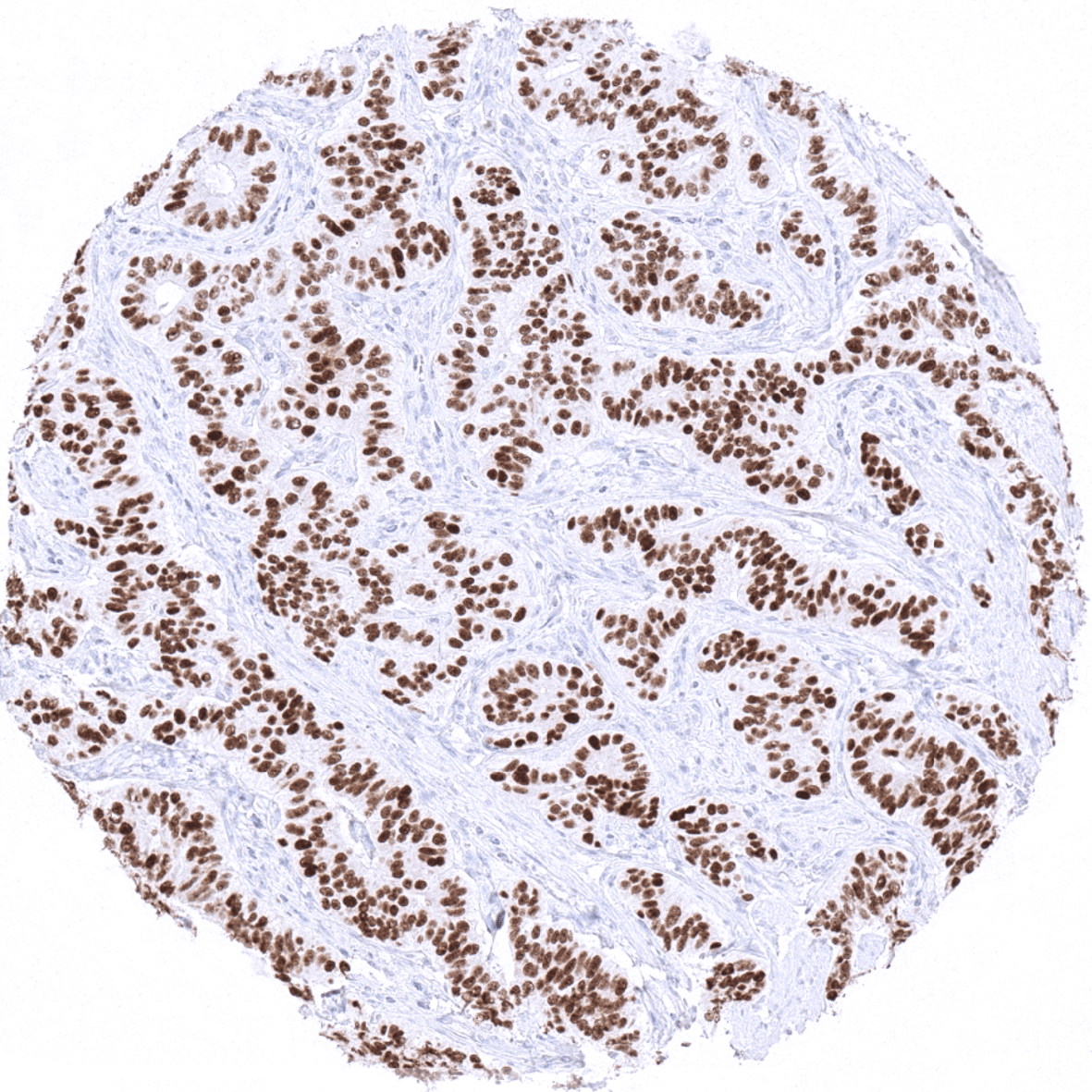
Positive control = Pancreas: Islet cells should show a moderate to strong INSM1 immunostaining
Negative control = Pancreas: All acinar cells should be negative
Cellular localization = Nuclear
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100
Intended Use = Research Use Only
Relevance of Antibody
Biology Behind
The Insulinoma-associated 1 (INSM1) is an intronless gene located at 20p11.23 coding for a 58kDa protein containing a zinc finger DNA-binding domain and a putative prohormone domain. It is primarily expressed in the developing nervous system and in neuroendocrine tissues. Its effects are caused by interactions with different molecules, depending on the type of tissue involved. INSM1 contributes to the induction of cell cycle arrest and exit necessary to facilitate cellular differentiation. The INSM1 protein can bind to both DNA and protein. Binding to cyclin D1 results in an inhibition of cell cycle progression. As a DNA binding transcription factor it for example represses transcription of NeuroD/β2 and the insulin gene in islet cells of the pancreas. Other known interaction partners of INSM1 include N-myc. RACK1, CDK4, Ngn3, NeuroD1, Phox2b and Mash 1N.
Staining Pattern in Normal Tissues
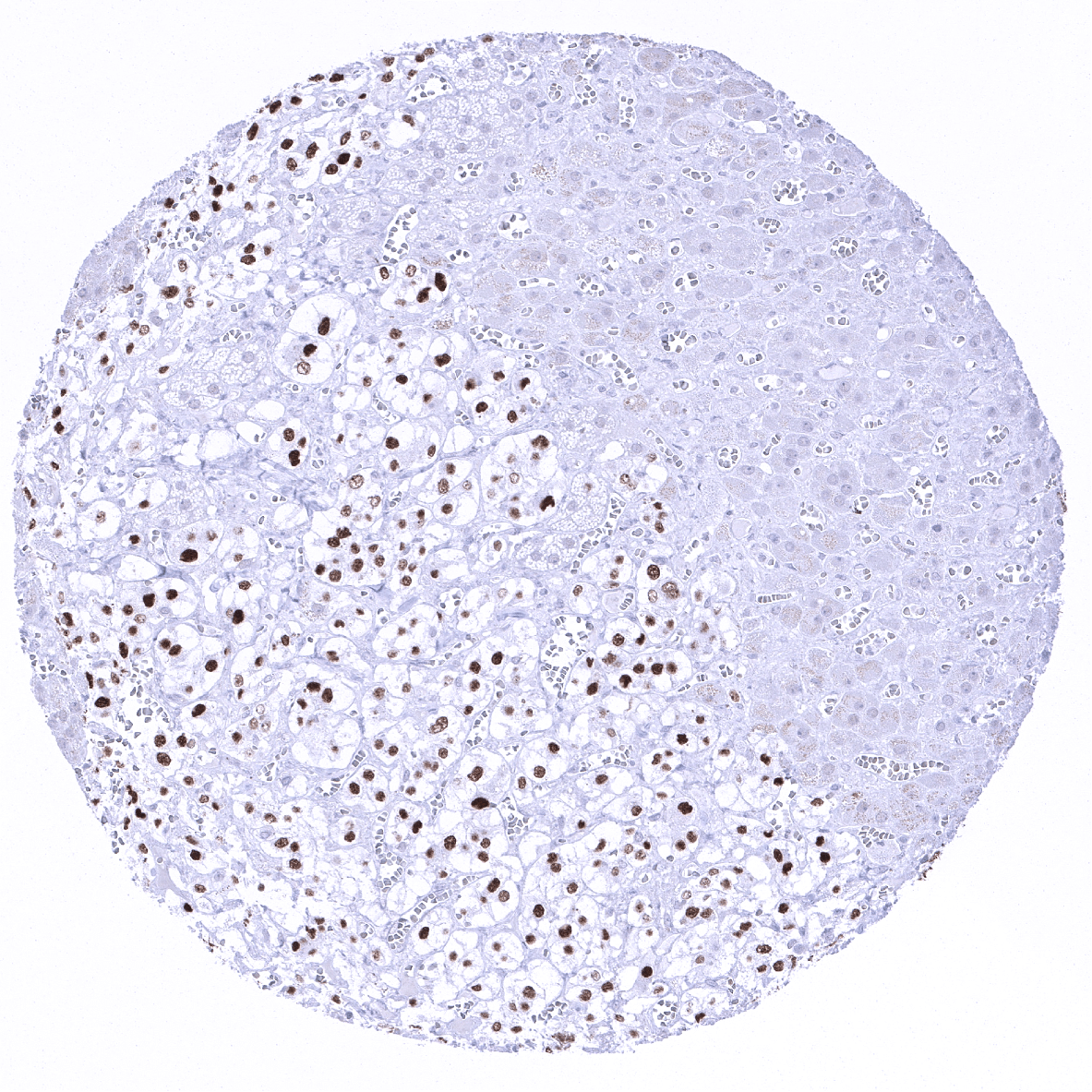
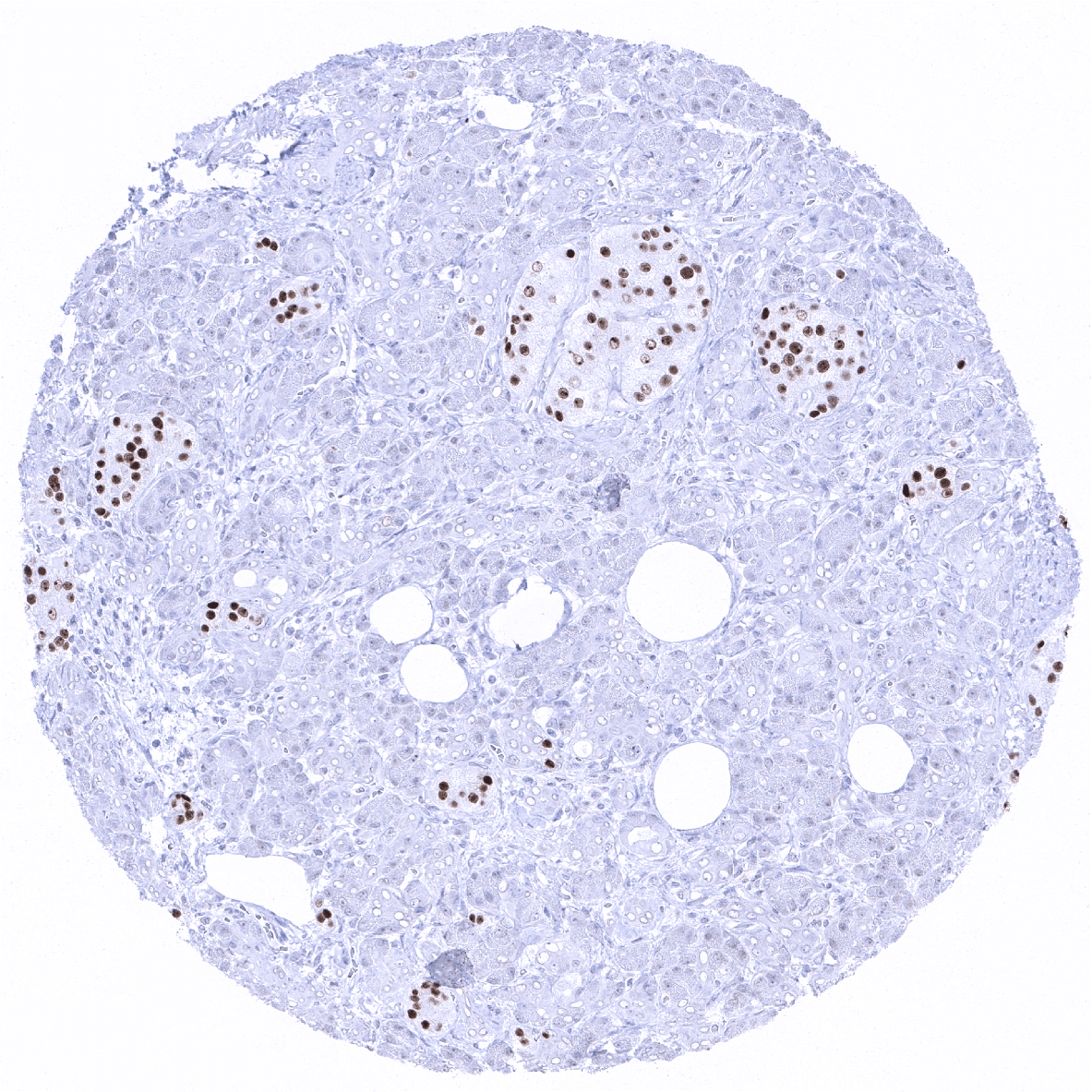
A moderate to strong INSM1 expression is seen in medullary cells of the adrenal gland, Islet cells of the pancreas, a fraction of cells in the adenohypophysis, scattered neuroendocrine cells in the colorectum, small intestine and the stomach, Few basally located – possibly also neuroendocrine – cells in the respiratory epithelium of the bronchus, and few scattered thymic epithelial cells in the thymic medulla.
These findings are to a large extent consistent with the RNA data summarized in the Human Protein Atlas (Tissue expression INSM1). While protein data are lacking in the protein atlas, the images on this web page describe the cell types responsible for INSM1 expression in several organs. However, brain cells expressing INSM1 protein were not detected. INSM1 expression in the brain may preferably occur during brain development.
Suggested positive tissue control: Pancreas: Islet cells should show a moderate to strong INSM1 immunostaining.
Suggested negative tissue control: Pancreas: All acinar cells should be negative.
Staining Pattern in Relevant Tumor Types
INSM1 is expressed in all kinds of neuroendocrine tumors, including neuroendocrine tumors and neuroendocrine cancers of various organs of origin, pheochromocytoma, paraganglioma, medullary thyroid cancer, Merkel cell carcinoma, and small cell carcinomas of various origins. INSM1 is also expressed in myxoid chondrosarcoma.
The TCGA findings on INSM1 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
INSM1 (MSVA-456R) publication summary
Relevant publication: Möller et al. “Comparison of INSM1 immunostaining with established neuroendocrine markers synaptophysin and chromogranin A in over 14,000 neuroendocrine and non-neuroendocrine tumors” Published in Mol Cell Endocrinol. 2024 Feb 1;581:112106. PMID: 37951531
A total of 12,286 tumors from 117 different tumor categories were successfully analyzed by using the following protocol: Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. MSVA-456R at a dilution of 1:100 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent). This protocol was also used for all stainings depicted in our tumor and normal tissue galleries.
INSM1 positivity was seen in 89.2% of 471 neuroendocrine neoplasms including 16.6% with weak, 22.3% with moderate, and 50.3% with strong staining. In non-neuroendocrine tumors, INSM1 staining occurred in 3.5% of 11,815 tumors including 2.2% with weak, 0.9% with moderate and 0.3% with strong positivity. Among 102 analyzed non-neuroendocrine tumor entities, INSM1 positivity was seen in at least one case in 59 different tumor categories, of which 15 entities contained at least one case with strong INSM1 positivity. The distribution of positive staining results is shown in an “organ-systematic” (Figure 1) and in a “ranking order” figure (Figure 2) below (images based on data from Möller et al). Data on associations with histopathological and clinical parameters of tumor aggressiveness are also summarized below (Figure 3; based on data described by Möller et al).
Authors conclusions on diagnostic utility of INSM1 IHC with respect to the distinction of different tumor entities (Möller et al.):
- INSM1 immunostaining is a sensitive and highly specific marker for neuroendocrine differentiation of tumors.
Authors conclusions on the prognostic role of INSM1 immunostaining results (Möller et al.):
- In breast carcinomas of no special type detectable INSM1 immunostaining was linked to low tumor stage (p = 0.0026), positive estrogen receptor (p =0.0004) and progesterone receptor status (p = 0.0025), but unrelated to overall survival (data not shown).
- INSM1 immunostaining was unrelated to histopathological features in neuroendocrine neoplasms, urothelial carcinoma and gastric adenocarcinomas.
Data from the publication: Möller et al. “Comparison of INSM1 immunostaining with established neuroendocrine markers synaptophysin and chromogranin A in over 14,000 neuroendocrine and non-neuroendocrine tumors” Published in Mol Cell Endocrinol. 2024 Feb 1;581:112106. PMID: 37951531
Summarized in own graphics:
Figure1. INSM1 staining in tumors (“organ-specific ; according to Möller et al.”) with antibody MSVA-456R
Figure 2. INSM1 staining in tumors (“ranking-order ; according to Möller et al.”) by positivity with antibody MSVA-456R.
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein. Accordingly, multiple different protocols can generate identical staining results.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
-Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH7,8 Target Retrieval Solution buffer. Apply MSVA-456R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Potential Research Applications
- INSM1 has been suggested as a sensitive marker of neuroendocrine differentiation and of neuroendocrine tumors but large-scale studies are lacking. Many tumor entities have not yet been analyzed for INSM1 expression.
- The prognostic and predictive role of INSM1 expression needs to be evaluated.
- The role of INSM1 as a therapeutic target is under investigation.
Evidence for Antibody Specificity in IHC
Utility of MSVA-456R is documented by a staining pattern that exactly matches the data summarized in the protein atlas. As suggested by the Human Protein Atlas (Tissue expression INSM1) data, a strong positive INSM1 staining is seen in neuroendocrine cells of various organs including medullary cells of the adrenal gland, Islet cells of the pancreas, a fraction of cells in the adenohypophysis as well as scattered neuroendocrine cells in the colorectum, small intestine and the stomach. At the same time, INSM1 staining is not found in tissues known to not express INSM1 including those notorious for non-specific IHC background such as kidney, colonic mucosa and epidermis.