295,00 € – 995,00 €
Product details
Synonyms = CD5 antigen (p56 62), LEU1, Ly12, LyA, Lymphocyte antigen T1/Leu-1, Lymphocyte glycoprotein T1/Leu1, T-cell surface glycoprotein CD5
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-005R
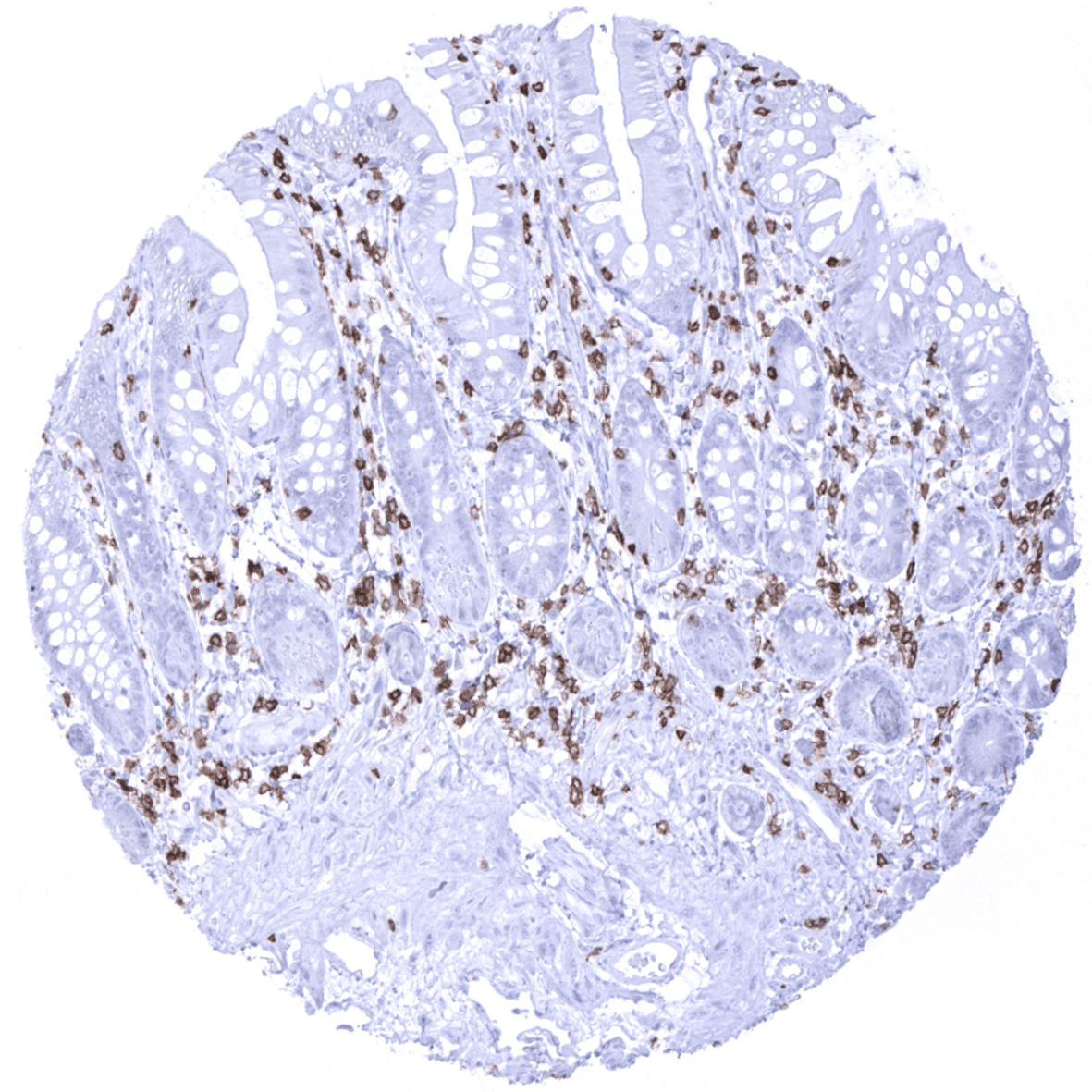
Positive control = Tonsil: A strong CD5 staining of virtually all T-cells in both T-zones and in germinal centres as well as an at least weak to moderate staining of dispersed B-cells in the mantle zone should be seen.
Negative control = Tonsil: CD5 staining should be absent in germinal centre B-cells and in surface epithelial cells (A fraction of crypt epithelial cells may stain positive).
Cellular localization = Cell surface
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
CD5 is expressed on pre-naive B-lymphocytes and most T-lymphocytes.
Biology Behind
CD5 (syn. Leu-1) is a cluster of differentiation protein located on the surface of the vast majority of T-lymphocytes and in pre-naive B-lymphocytes. Because T-cells express much higher levels of CD5 than B-cells, antibodies against CD5 were initially used as T-cell markers. CD5+ B-cells represent about 1–8% of peripheral blood lymphocytes and 5–30% of the circulating B-cells. Both in T- and B-lymphocytes, the function of CD5 is not fully understood. In B-lymphocytes, CD5 may mitigate activating signals from the B-cell receptor so that the CD5 positive B-cells can only be activated by very strong stimuli (such as bacterial proteins) and not by normal tissue proteins. In T-cells, the functional role of CD5 may vary between immature (thymic) and mature peripheric cells. In mature T-cells, the co-receptor CD5 may act as a negative regulator of T-cell activation, perhaps in a similar way as CTLA-4 and PD-1. Despite its unknown function, immunohistochemical CD5 staining has proven highly useful in the diagnosis of B-CLL and mantle cell lymphoma (mostly CD5 positive).
Staining Pattern in Normal Tissues
CD5 immunostaining occurs on the surface membranes of the vast majority of T-cells and in pre-naive B-cells mainly occurring in the mantle zone of germinal centres. A positive immunostaining is also seen in several epithelial tissues including a fraction of squamous epithelial cells lining tonsillar crypts, intercalated ducts in submandibular gland, bronchial glands, and – at least in a fraction of samples – surface epithelium in the stomach antrum.
These findings are largely comparable to the data described in the Human Protein Atlas (Tissue expression CD5)
Positive control: Tonsil: A strong CD5 staining of virtually all T-cells in both T-zones and in germinal centres as well as an at least weak to moderate staining of dispersed B-cells in the mantle zone should be seen.
Negative control: Tonsil: CD5 staining should be absent in germinal centre B-cells and in surface epithelial cells (A fraction of crypt epithelial cells may stain positive).
Staining Pattern in Relevant Tumor Types
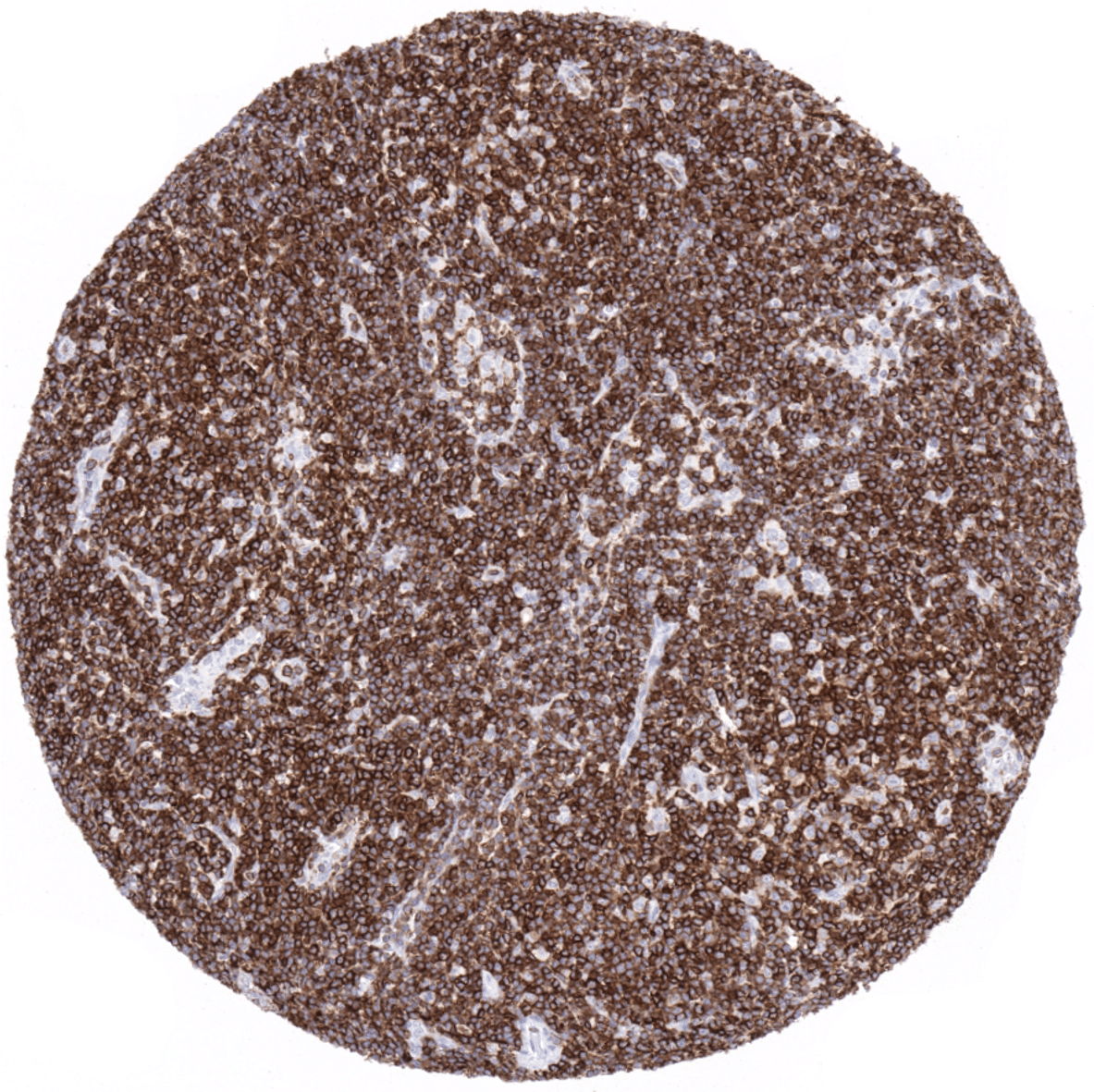
CD5 is strongly expressed in the vast majority of B-CLL, mantle cell lymphomas and T-cell lymphomas. CD5 expression can, at markedly lower frequency also occur in other B-cell lymphomas. CD5 expression can be lost in T-cell lymphomas. Of note, a membranous CD5 staining can – rarely – also be found in various epithelial cancers such as for example colorectal cancer.
The TCGA findings on CD5 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
-Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 9 Target Retrieval Solution buffer. Apply MSVA-005R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
-Impact of pH
MSVA-005R results in strongest staining if pH9,0 is used for slide pretreatment. pH7,8 is acceptable but lower pH results in a significant reduction of sensitivity.
Potential Research Applications
- The regulatory role of CD5 in both B- and T- lymphocytes is not fully understood.
- CD5 should be used as a component of multicolor assays analyzing the role of lymphocyte subsets in cancer and other diseases.
- The biological and diagnostic significance of CD5 immunostaining in hematological and non- hematological neoplasms should be further investigated.
Evidence for Antibody Specificity in IHC
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
Orthogonal validation: For the antibody MSVA-005R suggested is documented by the strong concordance of the immunostaining with RNA expression data derived from the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project which are all summarized in the Human Protein Atlas (Tissue expression CD5) Immunostaining by using MSVA-005R was predominantly detected in lymphatic tissues for which RNA expression was also documented as well as in scattered lymphocytes in other organs.
Comparison of antibodies: A positive immunostaining was also found in non-lymphatic tissues including a fraction of squamous epithelial cells lining tonsillar crypts, intercalated ducts in submandibular gland, bronchial glands, and – at least in a fraction of samples – surface epithelium in the stomach antrum. These structures constitute small subsets of the total amount of cells in these organs and may have been largely underrepresented and thus missed in RNA analyses. True expression of MSVA-005R in these tissues is suggested by similar data obtained by the independent CD5 antibody HPA043416 (protein atlas) in:
-and in tonsil krypts
Moreover, no staining was seen in tissues notorious for non-specific IHC background such as kidney, colonic mucosa, and epidermis.