295,00 € – 995,00 €
Product details
Synonyms = CD163, CD163 antigen, Macrophage-associated antigen, M130, CD163 molecule, Hemoglobin scavenger receptor, MM130; Scavenger receptor cysteine rich type 1 protein M130
Antibody type = mouse monoclonal / IgG2
Clone = MSVA-163M
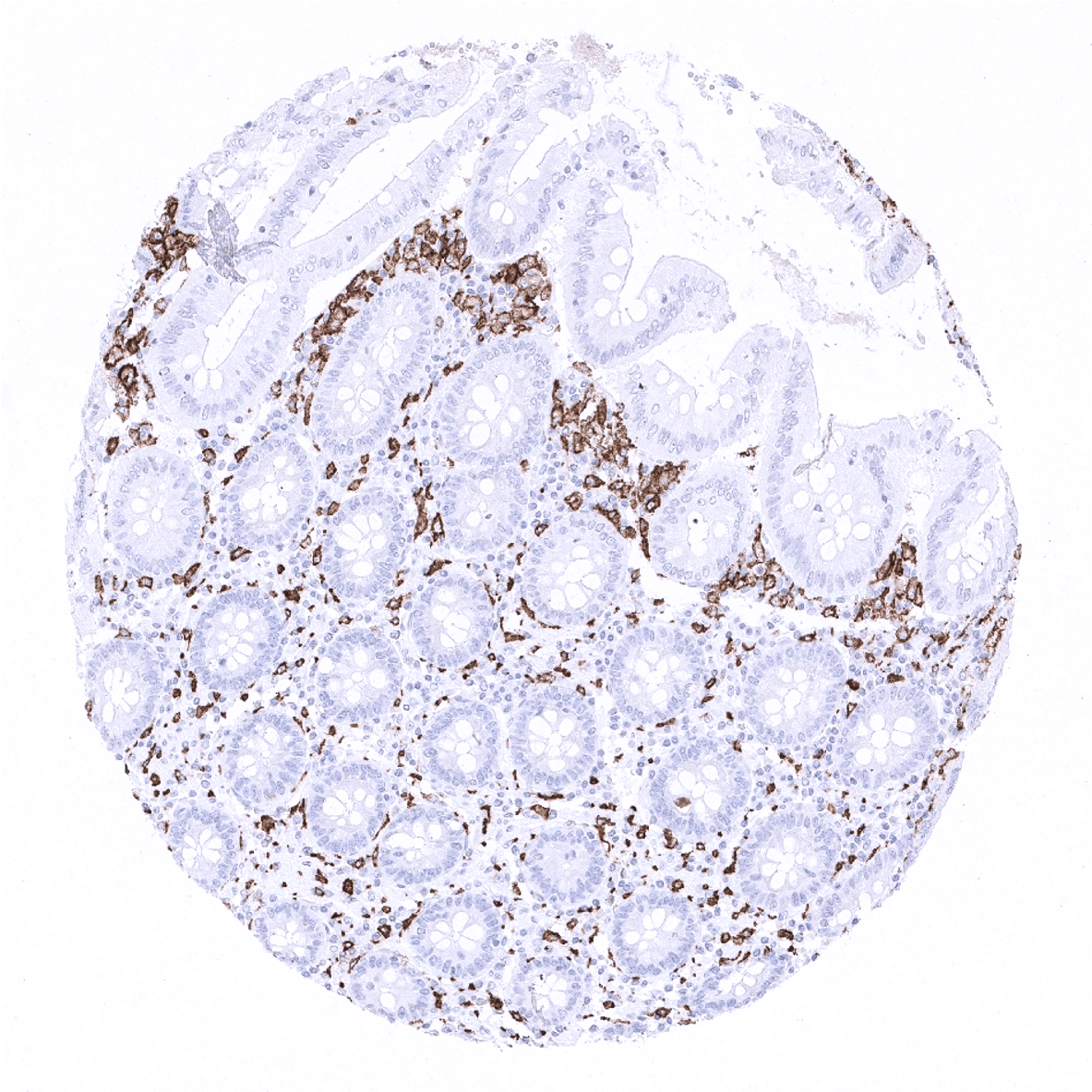
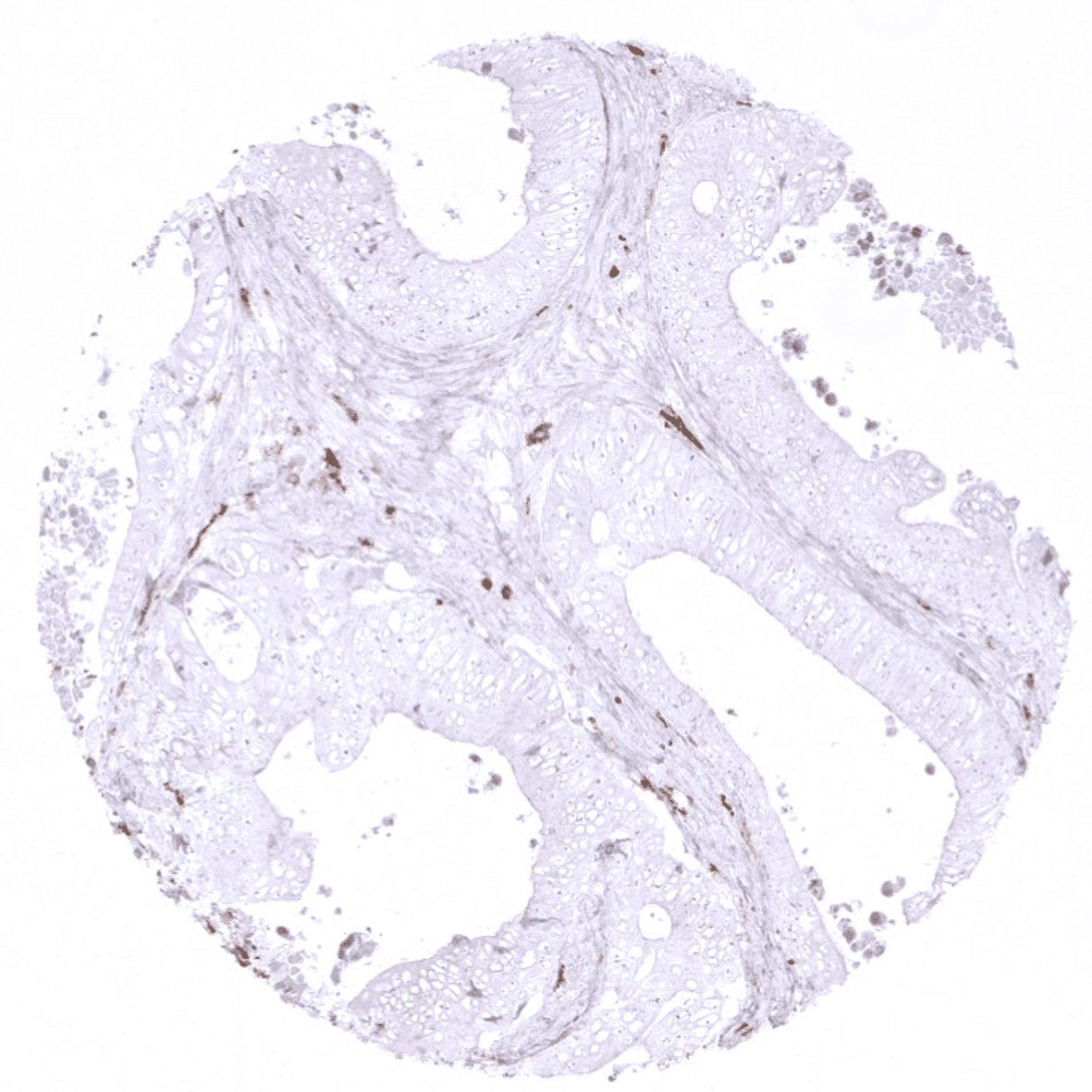
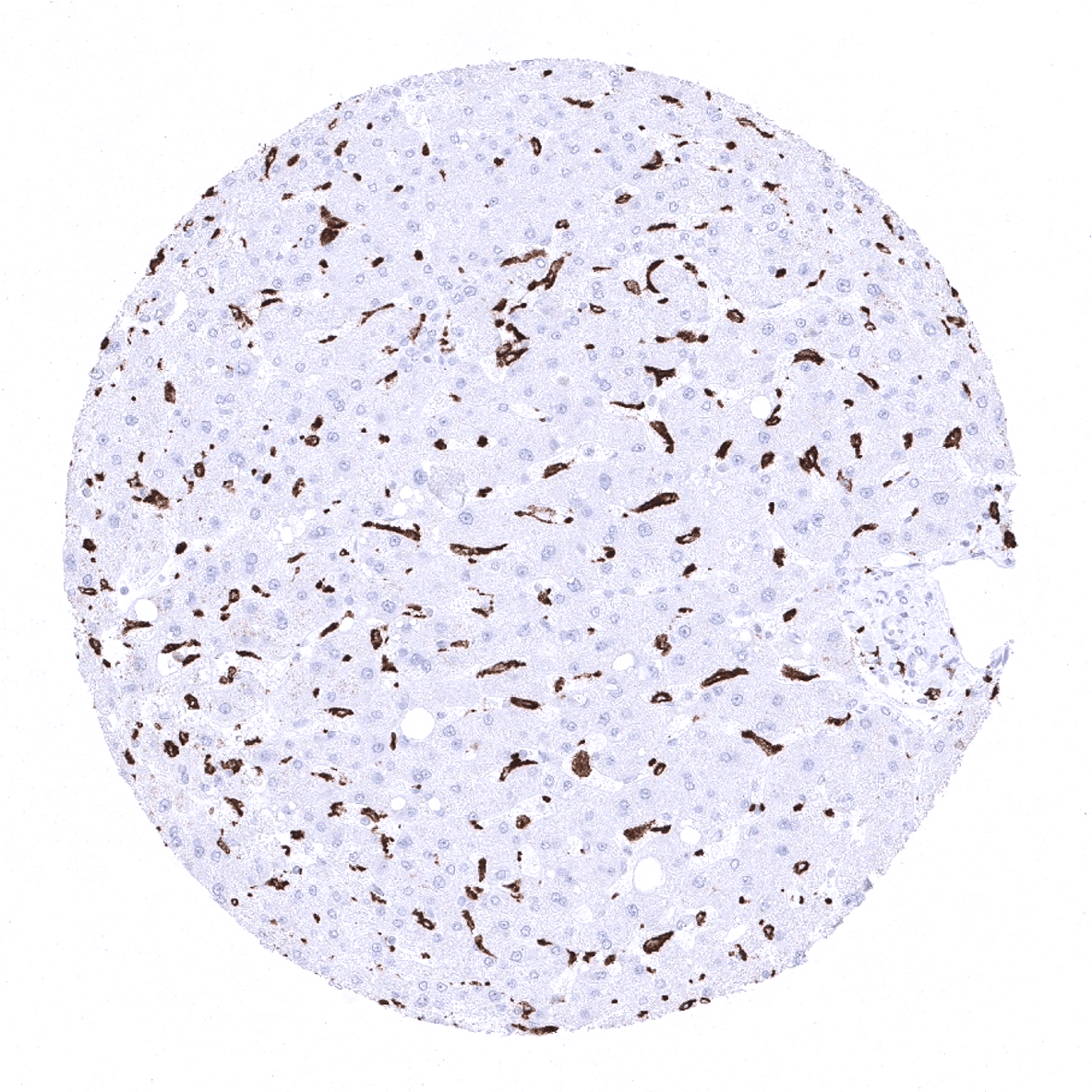
Positive control = A moderate to strong cytoplasmic staining of macrophages should be seen in the interfollicular zones of the tonsil, lamina propria of the appendix and in the Kupffer cells of the liver (see images). Macrophages surrounding the vessels in brain specimens should stain positive, while microglial cells should not show CD163 staining.
Negative control = CD163 staining should not be seen in hepatocytes and in epithelial cells of the appendix and tonsil.
Cellular localization = Membranous and cytoplasmic
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
Biology Behind
CD163 (Cluster of Differentiation 163) is a high affinity scavenger receptor for the hemoglobin-haptoglobin complex and in the absence of haptoglobin – with lower affinity – for hemoglobin alone. CD163 also functions as an innate immune sensor for gram-positive and gram-negative bacteria and serves as a marker of cells from the monocyte/macrophage lineage. Studies indicate that high level CD163 expression is a feature of macrophages undergoing differentiation toward the “alternatively activated” M2 phenotype. Compared to CD68 that is commonly used as a pan-macrophage marker, CD163 is thus regarded as a specific monocyte/macrophage marker for M2 macrophages. M2 macrophages have various different functions, including regulation of immunity, maintenance of tolerance, and tissue repair/wound healing. Presence of CD163+ macrophages was suggested to have a stronger association with unfavorable clinic-pathological cancer features than CD68+ macrophages. CD163 can also be shedded as soluble CD163 (sCD163) by inflammatory stimuli. Assessment of sCD163 in plasma, cerebrospinal fluid, and urine has shown clinical utility in several inflammatory diseases including for example sepsis, HIV infection, rheumatoid arthritis, systemic lupus erythematosus and ANCA vasculitis.
Staining Pattern in Normal Tissues
CD163 is expressed by all circulating monocytes and a majority of macrophages in tissues. CD163 positivity is for example seen in dendrocytes of the spleen, alveolar macrophages of the lung and Kupffer cells of the liver. In lymphatic tissues, CD163 is lacking in macrophages of the mantle zone, a large fraction of macrophages of the germinal center cells in lymph follicles, Langerhans cells and interdigitating reticulum cells.
These findings are fully consistent with the data and images presented in the Human Protein Atlas (Tissue expression CD163).
Suggested positive tissue control: A moderate to strong cytoplasmic staining of macrophages should be seen in the interfollicular zones of the tonsil, lamina propria of the appendix and in the Kupffer cells of the liver (see images). Macrophages surrounding the vessels in brain specimens should stain positive, while microglial cells should not show CD163 staining.
Suggested negative tissue control: CD163 staining should not be seen in hepatocytes and in epithelial cells of the appendix and tonsil.
Staining Pattern in Relevant Tumor Types
The vast majority of cancers contain CD163 positive macrophages. Their number and tissue distribution is highly variable, however. For example, CD163 positive macrophages can be limited to the cancer stroma or predominantly be located in direct contact to tumor cells. In some cases CD163 positive macrophages accumulate at the periphery of nests or sheets composed of cancer cells. Examples of CD163 immunostainings of tumors are shown in the cancer image gallery.
The TCGA findings on CD163 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein. Accordingly, multiple different protocols can generate identical staining results.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (more than 10 days between cutting and staining can lead to weaker staining results). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 9. Primary antibody specific against CD163 ( MSVA-163M, dilution 1:150) is applied at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-163M 1:150 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-163M 1:150 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Roche – Ventana Discovery ULTRA
Pretreatment for 64 minutes at 100°C (pH 8,4); CM peroxidase blocking for 12 minutes (room temperature), MSVA-163M 1:50 for 20 minutes at 36°C, secondary antibody (anti-mouse HQ) for 12 minutes at 36°C, anti-HQ HRP for 12 minutes at room temperature, DAB at room temperature, hematoxylin II at room temperature for 8 minutes, bluing reagent at room temperature for 4 minutes.
These images depict staining results obtained by the protocol described above. It is of note, that the Ventana machines generally require higher antibody concentrations than other commonly used autostainers because the antibodies are automatically diluted during the procedure. Various other protocols can result in an identical result as shown above. A longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of secondary antibody and or the anti-HQ HRP result in stronger staining, potentially at the cost of more background staining.
Impact of pH
MSVA-163M stains almost identically for pH6 to 9
Potential Research Applications
- CD163 is of very high research interest as it represents an important component of inflammatory processes in oncological and inflammatory disease.
- CD163 is a marker for M2 macrophages, the relevance of which needs to be further investigated.
- The prognostic relevance of the number of CD163 positive cells in cancer is not well known.
Evidence for Antibody Specificity in IHC
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. Comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
Orthogonal validation: For the antibody MSVA-163M, immunostaining data were compared with data from three independent RNA screening studies, including the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project, which are all summarized in the Human Protein Atlas (Tissue expression CD163). In agreement with RNA expression data, CD163 positive cells were particularly common in the lung, liver, spleen, lymph node and the appendix. The existence of at least few CD163 positive cells in virtually all normal tissues is reflected by at least some RNA expression documented in every organ. This, however, limits the potential of orthogonal validation for this antibody.
Comparison of antibodies: CD163 specific staining of MSVA-163M is corroborated by comparison with a commercially available independent second antibody (termed “validation antibody”). Both antibodies showed identical staining patterns in lymph node, myometrium, kidney pelvis, chorion, liver, heart, pancreas, colon, endometrium, cerebellum, adrenal gland, placenta (comparative images shown below). and in all other tissues. Specificity of MSVA-163M is further corroborated by a strong positive staining in cell types that are well documented to express CD163 such as tissue macrophages including Kupffer cells of the liver and alveolar macrophages of the lung.
Antibody Comparison: MSVA-163M vs another commercially available CD163 antibody called “Validation Antibody”