295,00 € – 995,00 €
Product details
Synonyms = Arginase 1; ARG1; liver-type arginase; type I arginase
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-511R
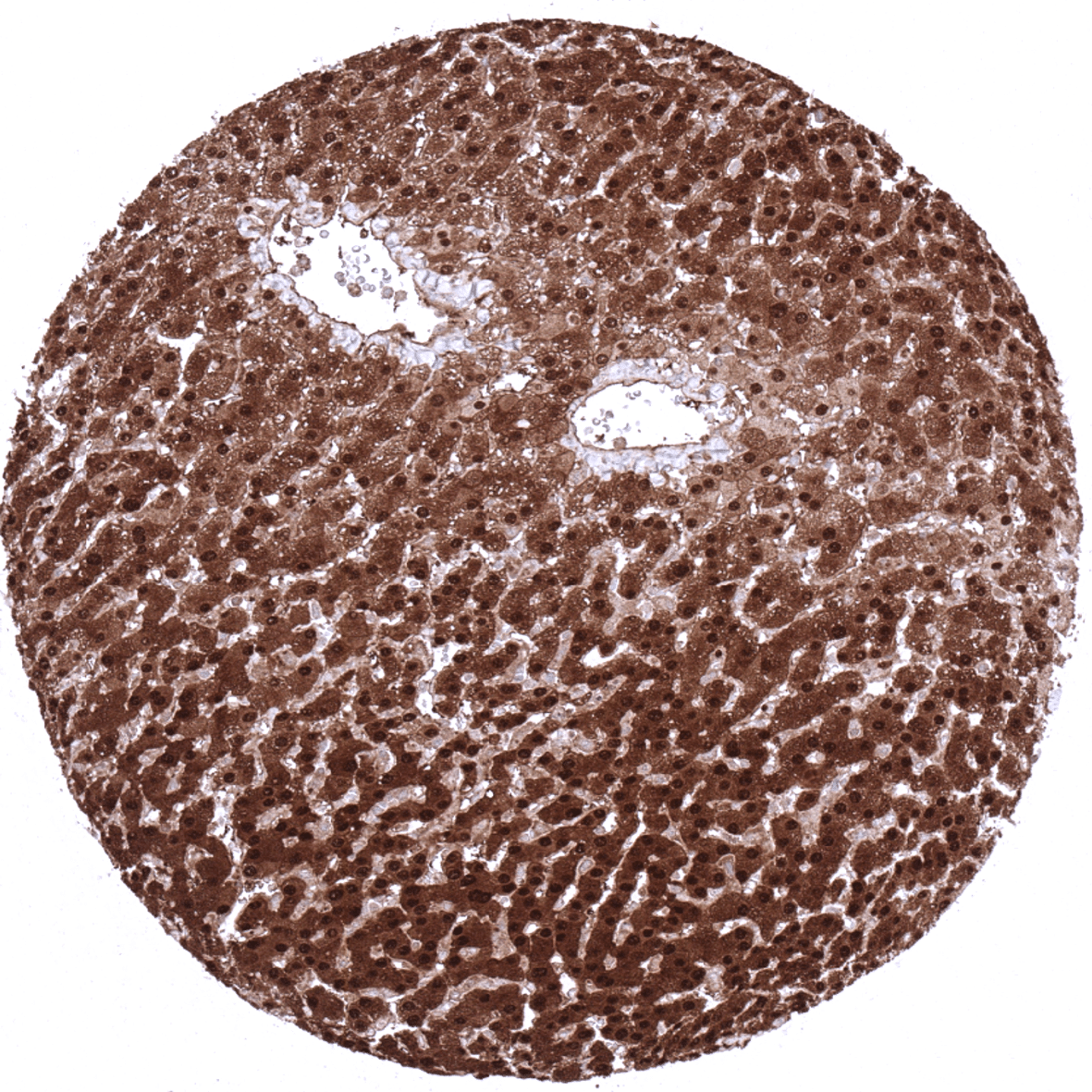
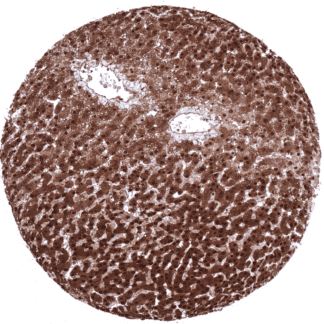
Positive control = Liver: A strong staining should be seen in hepatocytes. Spleen: A moderate t strong positivity should be seen in granulocytes.
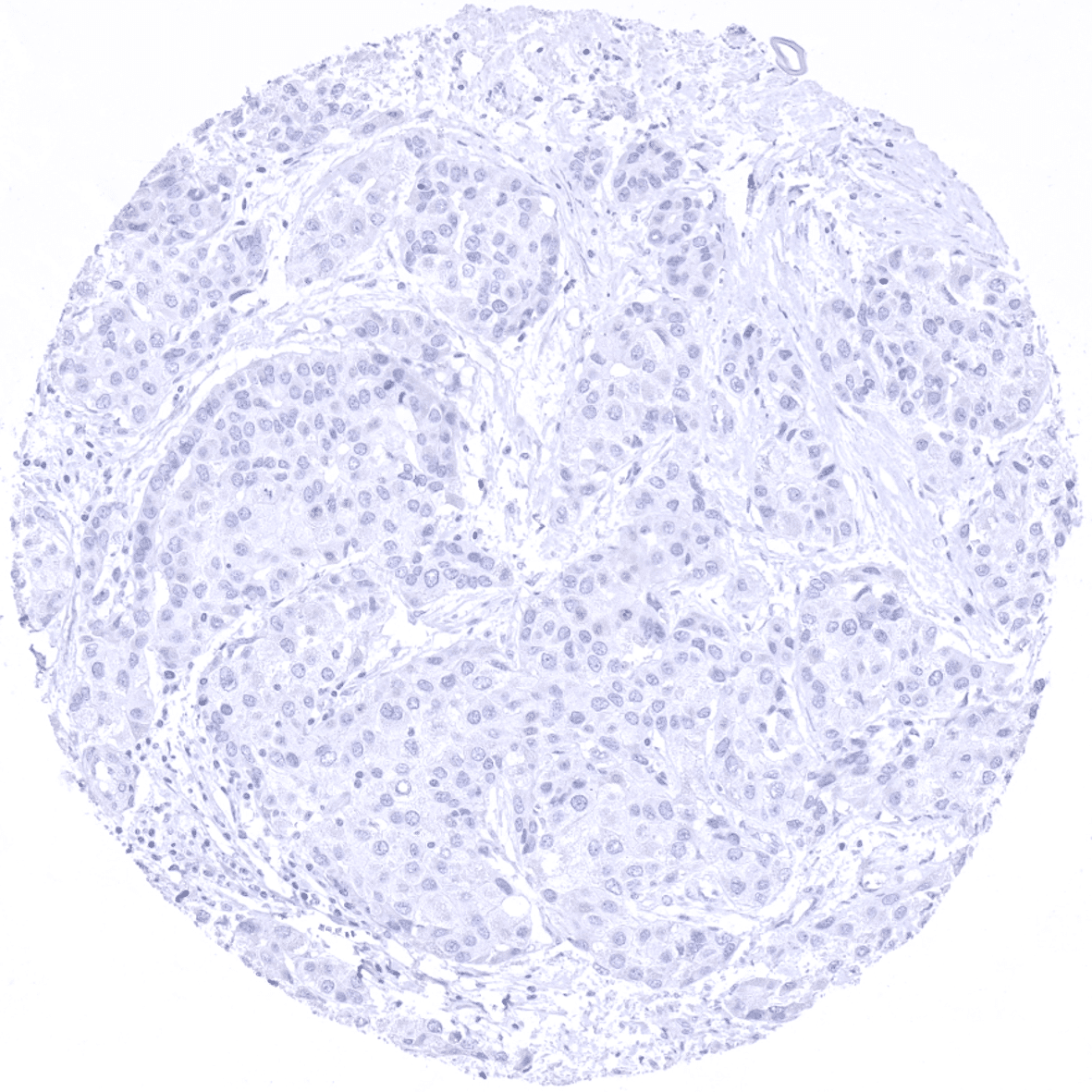
Negative control = Colon: Epithelial cells, smooth muscle and the vast majority of stroma cells should stain negative (few granulocytes may stain positive).
Cellular localization = Nuclear and cytoplasmic.
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
Arginase 1 is expressed in hepatocytes.
Biology Behind
Arginase-1 is coded by the ARG1 gene located at 6q23. It acts as an enzyme that catalyzes the conversion of arginine to ornithine and urea in the final step of the urea cycle. Inherited deficiency of both alleles of this enzyme results in argininemia, an autosomal recessive disorder characterized by hyperammonemia and resulting in progressive neurological disorder. [1]
[1] Lennartz et al. “Large-Scale Tissue Microarray Evaluation Corroborates High Specificity of High-Level Arginase-1 Immunostaining for Hepatocellular Carcinoma. Diagnostics” (Basel). 2021 Dec 14;11(12):2351. doi: 10.3390/diagnostics11122351. PMID: 34943588; PMCID: PMC8699869.
Staining Pattern in Normal Tissues
Arginase-1 staining pattern in Normal Tissues with antibody MSVA-511R (images are shown in our “Normal Tissue Gallery”)
| Brain | Cerebrum | Negative. |
| Cerebellum | Negative. | |
| Endocrine Tissues | Thyroid | Negative. |
| Parathyroid | Negative. | |
| Adrenal gland | Negative. | |
| Pituitary gland | Negative. | |
| Respiratory system | Respiratory epithelium | Negative. |
| Lung | Negative. | |
| Gastrointestinal Tract | Salivary glands | Negative. |
| Esophagus | Negative. | |
| Stomach | Negative. | |
| Colon | Negative. | |
| Duodenum | Negative. | |
| Rectum | Negative. | |
| Small intestine | Negative. | |
| Liver | The strongest nuclear and cytoplasmic Arginase-1 staining occurs in hepatocytes. | |
| Gallbladder | Negative. | |
| Pancreas | Negative. | |
| Genitourinary | Kidney | Negative. |
| Urothelium | Negative. | |
| Male genital | Prostate | Negative. |
| Seminal vesicles | Negative. | |
| Testis | Negative. | |
| Epididymis | Negative. | |
| Female genital | Breast | Negative. |
| Uterus, myometrium | Negative. | |
| Uterus, ectocervix | Negative. | |
| Uterus endocervix | Negative. | |
| Uterus, endometrium | Negative. | |
| Fallopian Tube | Negative. | |
| Ovary | Negative. | |
| Placenta early | Negative. | |
| Placenta mature | Weak to moderate cytoplasmic Arginase-1 positivity in a fraction of decidua cells. | |
| Amnion | Negative. | |
| Chorion | Negative. | |
| Skin | Epidermis | Moderate to strong Arginase-1 positivity in the granular cell layer of keratinizing squamous epithelium. |
| Sebaceous glands | Negative. | |
| Muscle/connective tissue | Heart muscle | Negative. |
| Skeletal muscle | Negative. | |
| Smooth muscle | Negative. | |
| Vessel walls | Negative. | |
| Fat | Negative. | |
| Stroma | Negative. | |
| Endothelium | Negative. | |
| Bone marrow/lymphoid | Bone marrow | Moderate Arginase-1 staining in granulocytes. |
| Lymph node | Negative. | |
| Spleen | Moderate Arginase-1 staining in granulocytes. | |
| Thymus | Negative. | |
| Tonsil | Negative. | |
| Remarks | Arginase-1 staining is usually cytoplasmic and nuclear. Granulocytes (arginase-1 positive) can occur in all tissues. |
These findings are largely comparable to the RNA data summarized in the Human Protein Atlas (Tissue expression Arginase-1) showing predominant arginase-1 RNA expression in the liver and much weaker expression in granulocytes and skin. It is not surprising that decidua cell positivity was not described in these RNA analyses. These cells constitute very small subsets of the total amount of cells in the placenta and may have been largely underrepresented in RNA analyses. It is not clear based on the findings obtained with our antibody, which cell types may be responsible for the RNA expression described in the lung (granulocytes?).
Suggested positive tissue control: Liver: A strong staining should be seen in hepatocytes. Spleen: A moderate t strong positivity should be seen in granulocytes.
Suggested negative tissue control: Colon: Epithelial cells, smooth muscle and the vast majority of stroma cells should stain negative (few granulocytes may stain positive).
Staining Pattern in Relevant Tumor Types
Arginase-1 is expressed in the vast majority of hepatocellular carcinomas but occurs only exceptionally in other tumors. A focal arginase positivity can occasionally be seen in specific cell layers of squamous cell carcinomas reflecting the granulosa cell layer of normal skin. Arginase-1 expression has been reported to occur in hepatoid carcinoma of the pancreas.
The TCGA findings on Arginase-1 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
Arginase-1 (MSVA-511R) publication summary
Relevant publication: Lennartz et al. “Large-Scale Tissue Microarray Evaluation Corroborates High Specificity of High-Level Arginase-1 Immunostaining for Hepatocellular Carcinoma. Diagnostics”. Published in (Basel). 2021 Dec 14;11(12)
A total of 14912 tumors were analyzed from 117 different tumor categories by using the following protocol: Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH7,8 Target Retrieval Solution buffer. MSVA-511R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent). This protocol was also used for all stainings depicted in our tumor and normal tissue galleries.
A nuclear and cytoplasmic arginase-1 immunostaining was predominantly observed in hepatocellular carcinoma, where 96% of 49 cancers were at least moderately positive. Although 22 additional tumor categories showed occasional arginase immunostaining, strong staining was exceedingly rare in these entities. Staining of a few tumor cells was most commonly observed in squamous cell carcinomas of various sites. In these tumors, staining typically involved maturing cells with the beginning of keratinization. Accordingly, low level arginase-1 positivity was significantly associated with a low grade in 635 squamous cell carcinomas of various sites (p = 0.003). The distribution of positive staining results is shown “organ-systematic” and in a “ranking order” figure below (images based on data from Lennartz et al).
Authors conclusions on diagnostic utility with respect to the distinction of benign versus malignant (Lennartz et al.):
- not applicable.
Authors conclusions on diagnostic utility with respect to the distinction of different tumor entities (Lennartz et al.):
- A positive nuclear and cytoplasmic arginase-1 immunohistochemistry is highly sensitive and specific for hepatocellular carcinoma if weak and focal staining is disregarded.
Authors conclusions on prognostic/predictive role of Arginase-1 expression (Lennartz et al.):
- In squamous cell carcinomas, arginase-1 staining is significantly associated with a low grade
1. Arginase-1 staining in tumors “organ-specific” with antibody MSVA-511R
2. Arginase-1 staining in tumors “ranking order” by positivity with antibody MSVA-511R.
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply MSVA-511R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-511R 1:300 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-511R 1:150 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Roche – Ventana Discovery ULTRA
Pretreatment for 64 minutes at 100°C (pH 8,4); CM peroxidase blocking for 12 minutes (room temperature), MSVA-511R 1:100 for 20 minutes at 36°C, secondary antibody (anti-rabbit HQ) for 12 minutes at 36°C, anti-HQ HRP for 12 minutes at room temperature, DAB at room temperature, hematoxylin II at room temperature for 8 minutes, bluing reagent at room temperature for 4 minutes.
These images depict staining results obtained by the protocol described above. It is of note, that the Ventana machines generally require higher antibody concentrations than other commonly used autostainers because the antibodies are automatically diluted during the procedure. Various other protocols can result in an identical result as shown above. A longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of secondary antibody and or the anti-HQ HRP result in stronger staining, potentially at the cost of more background staining.
Potential pitfalls
Tissue located in the vicinity of highly positive normal liver tissue may show a weak to moderate staining of all cell types (epithelial cells and stroma) due to contamination derived from very high arginase-1 levels in adjacent normal tissues.
Potential Research Applications
- The expression of Arginase-1 in hepatic and extra-hepatic cancers needs to be evaluated to comprehensively assess the diagnostic utility of arginase-1 immunohistochemistry.
- The prognostic role of arginase expression levels in hepatocellular and squamous cell carcinomas are not clarified yet.
Evidence for Antibody Specificity in IHC
There are two ways, how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
For the antibody MSVA-511R specificity is documented by the strong concordance of the immunostaining with RNA expression data derived from the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project which are all summarized in the Human Protein Atlas (Tissue expression Arginase-1). The RNA data show predominant arginase-1 RNA expression in the liver and much weaker expression in granulocytes and skin. These observations largely correspond to the findings obtained by using MSVA-511R. It is not surprising that decidua cell positivity was not described in these RNA analyses. These cells constitute very small subsets of the total amount of cells in the placenta and may have been largely underrepresented in RNA analyses. It is not clear based on the findings obtained with our antibody, which cell types may be responsible for the RNA expression described in the lung (granulocytes?). True expression of arginase-1 in decidua cells is also supported by an image from the protein atlas depicting this very same finding by using the antibody HPA024006
True expression of arginase-1 in a specific (granular layer) cell layer of the skin is also confirmed by findings obtained by HPA024006.
Moreover, no staining was seen in tissues notorious for non-specific IHC background such as kidney and colonic mucosa.