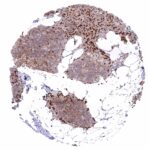
295,00 € – 995,00 €
Product details
Synonyms = nuclear factor I X , CTF , MRSHSS , NF-I/X , NF1-X , NF1A , SOTOS2
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = HMV329
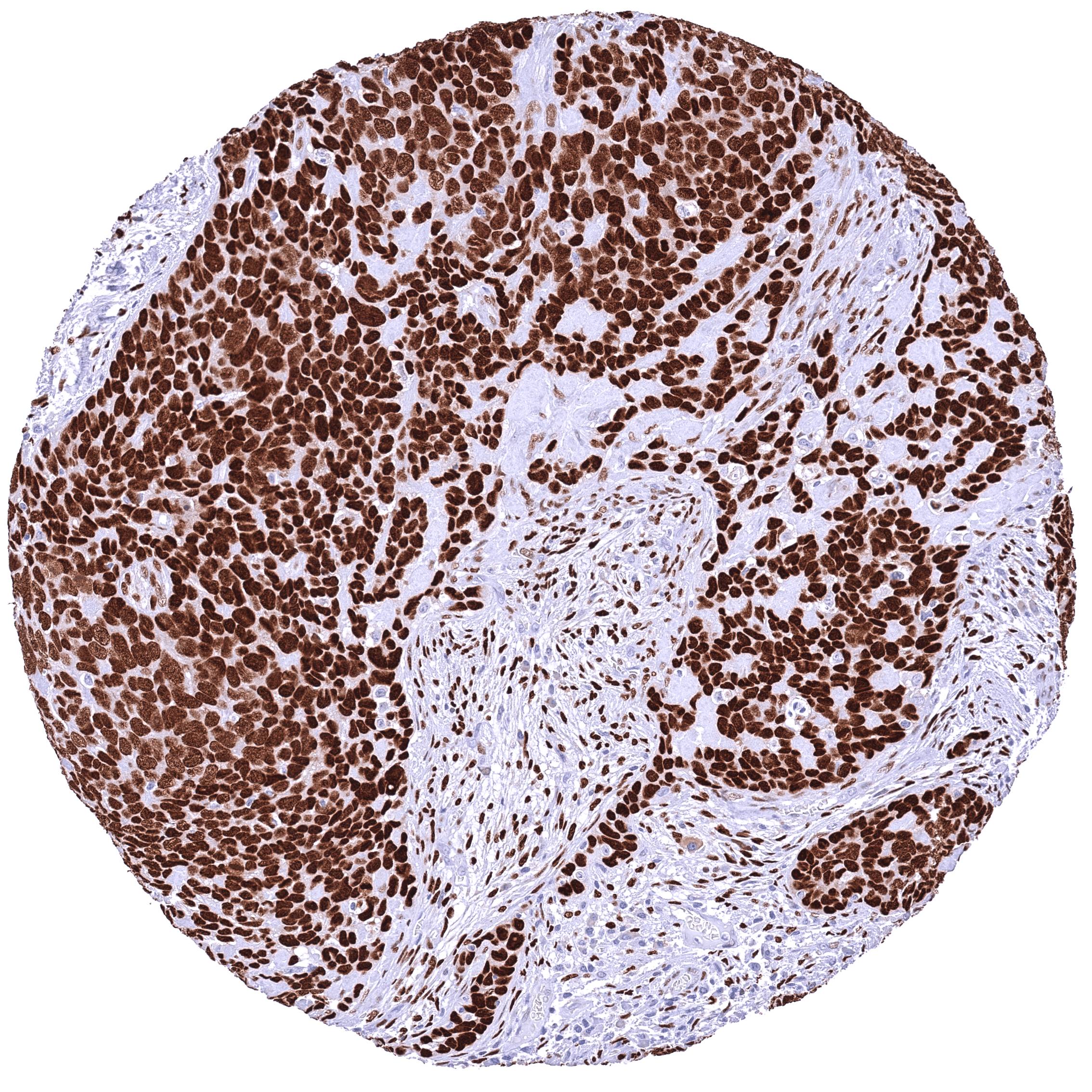
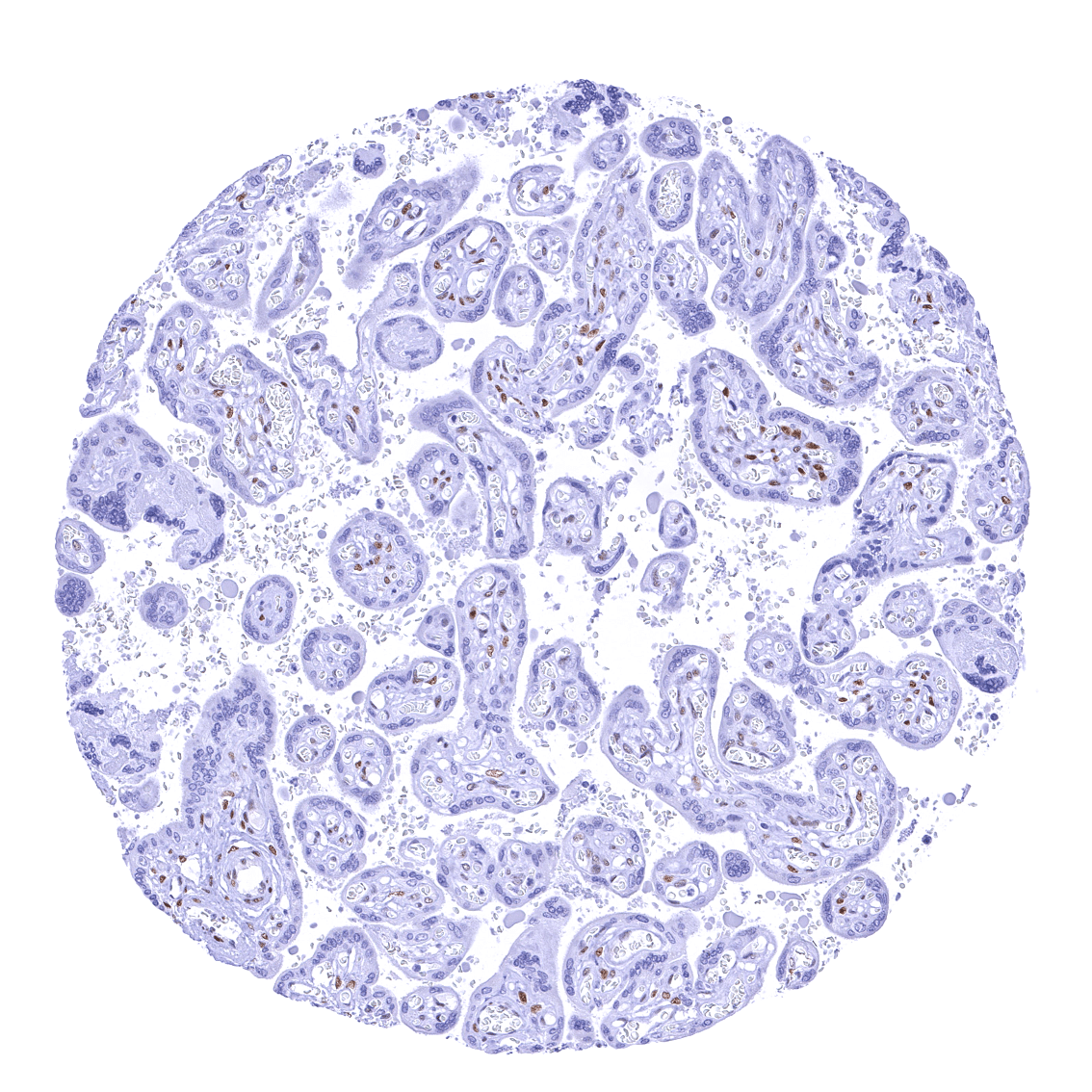
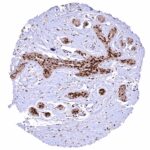
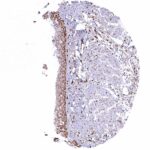
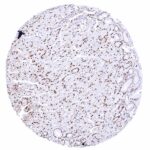
Positive control = Placenta: A moderate to strong nuclear NFIX staining should be seen in stromal cells while trophoblast cells should remain NFIX negative.
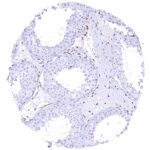
Negative control = Placenta: Trophoblast cells should remain NFIX negative while a moderate to strong nuclear NFIX staining should be seen in stromal cells.
Cellular localization = Nucleus
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
NFIX is a master transcription factor interacting with many other transcription factors and chromatin.
Biology Behind
Nuclear factor 1 X-type (NFIX) is a transcription factor protein which is coded by the NFIX gene on chromosome 19p13.13. Besides NFIA, NFIB, NFIC, NFIX is one out of four closely related members of the nuclear factor I (NFI) family of transcription factors. The family members share a particularly high rate of interactions with other transcription factors. They are therefore thought to not only directly regulate the expression of genes but also believed to modulate the function of other transcription factors. NFIX is known to play a role in muscle and central nervous system embryonic development. For example, NFIX has been shown to control the timing of neural differentiation promoting the ongoing growth of the hippocampus and proper memory function. The role of NFIX in other tissues is less intensively studied. Various types of NFIX alterations have been found in tumors. Mechanisms leading to both increased and reduced expression have been found to promote pro-tumorigenic functions, such as increased cell proliferation and migration and disturbed differentiation.
Staining Pattern in Normal Tissues
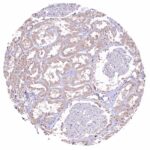
In normal tissues, NFIX is ubiquitously seen in nuclei of all tissues and most cell types. Images describing the NFIX staining pattern in normal tissues obtained by the antibody HMV329 are shown in our “Normal Tissue Gallery”.
| Brain | Cerebrum | Strong nuclear NFIX staining of glial cells. Weak or absent staining in neurons. |
| Cerebellum | Distinct nuclear NFIX staining of glial cells, moderate staining of granular cells, and lack of staining in Purkinje cells. | |
| Endocrine Tissues | Thyroid | Distinct nuclear NFIX staining of follicular cells. |
| Parathyroid | Moderate nuclear NFIX staining of all epithelial cells. | |
| Adrenal gland | Medulla with distinct nuclear NFIX staining of few interspersed stromal (sustentacular cells? endothelial?) cells while adrenal medullary cells remain NFIX negative. Weak to moderate staining of adrenocortical cells. | |
| Pituitary gland | Distinct nuclear NFIX staining of all pituicytes in the neurohypophysis while only few (epithelial??) cells of the adenohypophysis show a distinct nuclear NFIX staining. | |
| Respiratory system | Respiratory epithelium | Distinct nuclear NFIX staining of the cells of the respiratory epithelium, but the staining intensity decreases from the basal layer towards the more superficial cell layers. Strong nuclear NFIX staining of bronchial gland cells. |
| Lung | Strong nuclear NFIX staining of all cell types (except macrophages). | |
| Gastrointestinal Tract | Salivary glands | Strong nuclear NFIX staining of epithelial cells from glands and excretory ducts. |
| Esophagus | Distinct nuclear NFIX staining of all cells. Significant decrease of NFIX staining of squamous epithelium from the basal/suprabasal to the superficial cell layers. | |
| Stomach | Moderate to strong nuclear NFIX staining of most epitheliall cells but few glandular cells do only show faint or even absent staining. | |
| Duodenum | Nuclear NFIX staining is strong in Brunner glands, moderate in crypt bases and weak/faint or even absent in the surface epithelium. | |
| Small intestine | Nuclear NFIX staining is moderate in crypt bases and weak/faint or even absent in the surface epithelium. | |
| Appendix | Moderate to strong nuclear NFIX staining of epithelial cells. | |
| Colon | Weak to moderate to strong nuclear NFIX staining of epithelial cells. Slight but significant decrease of NFIX staining intensity from the crypt base to the superficial epithelial cell layers. | |
| Rectum | Weak to moderate to strong nuclear NFIX staining of epithelial cells. Slight but significant decrease of NFIX staining intensity from the crypt base to the superficial epithelial cell layers. | |
| Liver | Nuclear NFIX staining is largely absent in hepatocytes. NFIX staining is seen in stromal cells. | |
| Gallbladder | Nuclear NFIX staining is weak to moderate in epithelial cells but stronger in stromal cells. | |
| Pancreas | Strong nuclear NFIX staining of epithelial cells of excretory ducts and of intercalated ducts. NFIX staining is distinctively weaker and sometimes absent in acinar cells. | |
| Genitourinary | Kidney | Nuclear NFIX staining is generally low in all epithelial cell types. Staining is variable ranging from absent to faint/weak. |
| Urothelium | Distinct nuclear NFIX staining of urothelial cells but there is a significant decrease of NFIX staining intensity from the basal to the superficial cell layers. Umbrella cells are largely NFIX negative. | |
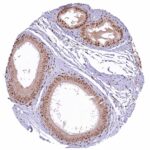
| Male genital | Prostate | Strong nuclear NFIX staining of stromal and of all epithelial cells. |
| Seminal vesicles | Strong nuclear NFIX staining of stromal and basal epithelial cells. NFIX staining is weak or absent in most luminal epithelial cells. | |
| Testis | Distinct nuclear NFIX staining of intertubular cells (including Leydig cells). Complete absence of NFIX staining in tubular cells (Sertoli cells, germ cells). | |
| Epididymis | Strong nuclear NFIX staining of epithelial cells in the cauda. In the caput, the NFIX staining is only faint or absent in chief cells but strong in basal cells. | |
| Female genital | Breast | Strong nuclear NFIX staining of a large subset of basal and luminal epithelial cells. |
| Uterus, myometrium | Strong nuclear NFIX staining of smooth muscle cells. | |
| Uterus, ectocervix | Distinct nuclear NFIX staining of squamous epithelial cells although there is a tendency towards a decreased staining intensity in the superficial cell layers. | |
| Uterus endocervix | Moderate nuclear NFIX staining of glandular cells. Strong nuclear NFIX staining of stromal cells. | |
| Uterus, endometrium | Strong nuclear NFIX staining of stromal cells while endometrium cells remain NFIX negative. | |
| Fallopian Tube | Strong nuclear NFIX staining of all cells. | |
| Ovary | Strong nuclear NFIX staining of stromal cells. Corpus luteum cells are NFIX negative. | |
| Placenta early | Strong nuclear NFIX staining of stromal cells while trophoblast cells remain negative. | |
| Placenta mature | Strong nuclear NFIX staining of stromal cells while trophoblast cells remain negative. | |
| Amnion | Negative. | |
| Chorion | Negative. | |
| Skin | Epidermis | Strong nuclear NFIX staining of all squamous epithelial cells. |
| Sebaceous glands | Weak to moderate nuclear NFIX staining of sebaceous gland cells. The staining intensity decreases towards the centre of the glands. | |
| Muscle/connective tissue | Heart muscle | Strong nuclear NFIX staining of all muscle cells. |
| Skeletal muscle | Strong nuclear NFIX staining of all muscle cells. | |
| Smooth muscle | Strong nuclear NFIX staining of all muscle cells. | |
| Vessel walls | Strong nuclear NFIX staining of endothelial and muscle cells. | |
| Fat | Strong nuclear NFIX staining of fat cells. | |
| Stroma | Variable (often strong) nuclear NFIX staining of various types of stroma cells. | |
| Endothelium | Strong nuclear NFIX staining of all endothelial cells. | |
| Bone marrow/ lymphoid tissue | Bone marrow | Strong nuclear NFIX staining of a rather small subset of cells (often arranged in groups). |
| Lymph node | Distinct nuclear NFIX staining of stromal and endothelial cells. Lymphocytes are NFIX negative. | |
| Spleen | Distinct nuclear NFIX staining of stromal and endothelial cells. Lymphocytes are NFIX negative. | |
| Thymus | Lymphocytes are NFIX negative. Distinct nuclear NFIX staining of epithelial cells. Maturing squamous epithelial cells of corpuscles of Hassall’s do only stain weakly or remain negative. | |
| Tonsil | Distinct nuclear NFIX staining of squamous epithelial cells although there is a tendency towards a decreased staining intensity in the superficial cell layers. Lymphocytes are largely negative. | |
| Remarks | The majority of cell types show a variable, often strong nuclear NFIX staining. |
These findings are largely comparable to the RNA and protein data described in the Human Protein Atlas (Tissue expression NFIX).
Positive control = Placenta: A moderate to strong nuclear NFIX staining should be seen in stromal cells while trophoblast cells should remain NFIX negative.
Negative control = Placenta: Trophoblast cells should remain NFIX negative while a moderate to strong nuclear NFIX staining should be seen in stromal cells.
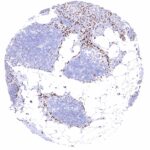
Staining Pattern in Relevant Tumor Types
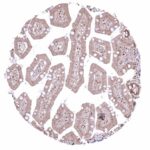
A variable level of NFIX staining is seen in many cases of most cancer types.
The TCGA findings on NFIX RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply HMV329 at a dilution of 1:200 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Potential Research Applications
- The role of NFIX in cancer is completely unclear.
- The patterns of NFIX protein expression in cancer have not been explored.
- The role of NFIX in heart disease is under investigation.
- The role of NFIX in other diseases is not specified yet.
Evidence for Antibody Specificity in IHC
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. Comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
Orthogonal validation: For the antibody HMV329, specificity is consistent by the consistency of the immunostaining data with data from three independent RNA screening studies, including the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project, which are all summarized in the Human Protein Atlas (Tissue expression NFIX). In agreement with HMV329 immunostaining data, NFIX RNA expression predominated in muscle tissues, was high in salivary glands, fallopian tube and the endometrium, and was low or absent in bone marrow, lymphatic tissues, kidney, liver and the testis. However, orthogonal validation is not optimal for proteins that are expressed in all organs.
Comparison of antibodies: True expression of NFIX in all cell types with nuclear NFIX positivity by HMV329 is corroborated by an identical nuclear staining obtained by a commercially available independent second antibody (termed “validation antibody”). Most of all, both antibodies showed several characteristic nuclear staining patterns such as strong staining in muscular tissue, ovarian stroma, epidermis, non-keratinizing squamous epithelium, respiratory epithelium, urothelium, excretory ducts and intercalated ducts of the pancreas, salivary epithelium, prostate epithelial cells, basal cells of the caput epididymis, stroma and Leydig cells of the testis, the lung, basal and luminal epithelial cells of the breast gland, endometrial stroma, fallopian tube, follicular cells of the thyroid, and in pituicytes. Absence of nuclear NFIX staining was confirmed by the validation antibody in umbrella cells, amnion, chorion, and trophoblast cells, lymphocytes, macrophages, most hematopoietic cells of the bone marrow, hepatocytes, Sertoli cells and germ cells of the testis, endometrium glands, corpus luteum, adrenal medullary cells, Purkinje cells of the cerebellum, and in most if not all cells of the adenohypophysis.
Independence of the validation antibody is demonstrated by a high level cytoplasmic cross-reactivity in a broad range of different cell types which was only seen by the and not by HMV329.