195,00 € – 745,00 €
Product details
Synonyms = Minichromosome Maintenance Complex Component 3, DNA Polymerase Alpha Holoenzyme-Associated Protein P1, DNA Replication Licensing Factor MCM3, RLF Subunit Beta, P1-MCM3, P102
Antibody type = Mouse monoclonal / IgG
Clone = MSVA-503M
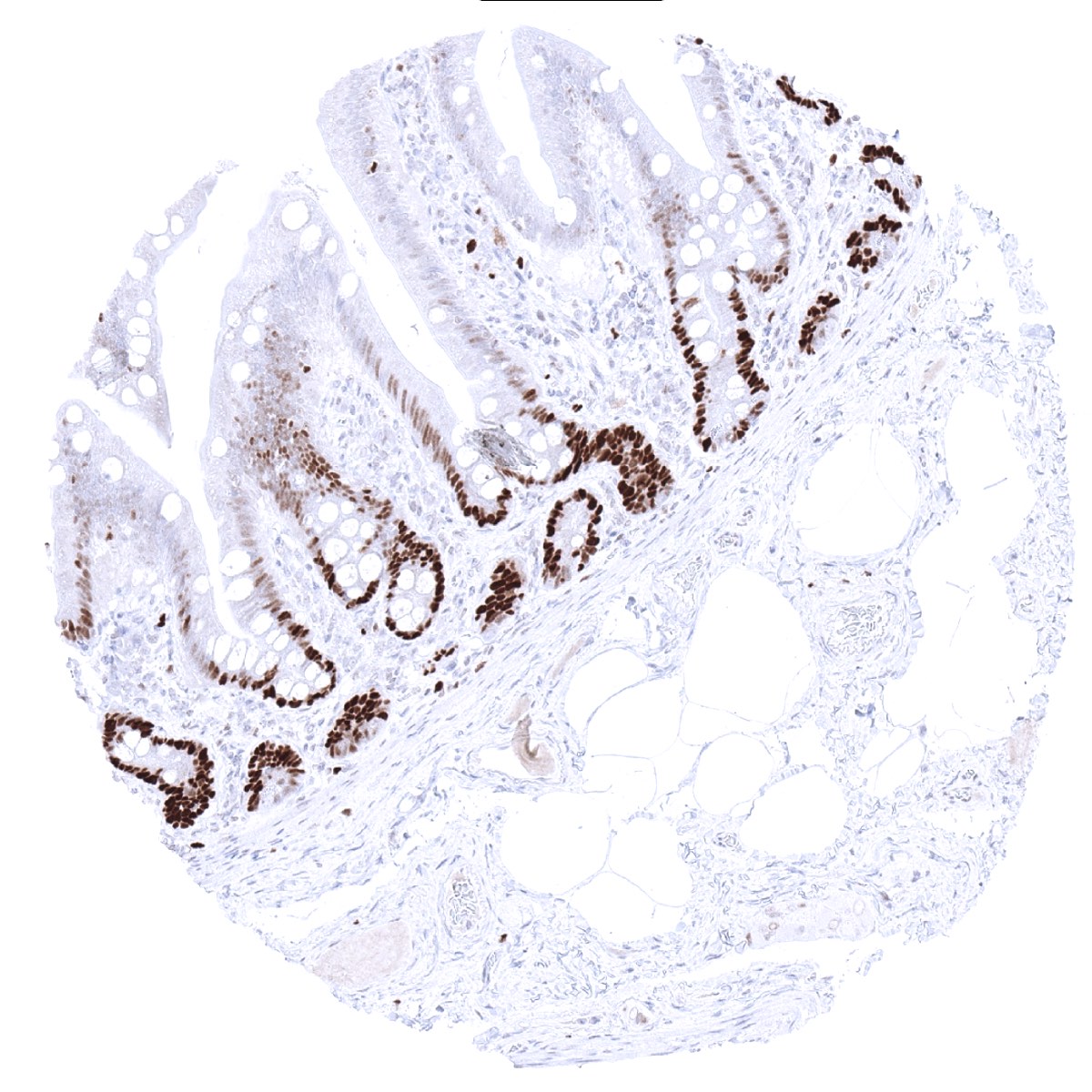
Positive control = Colon: A strong nuclear MCM3 immunostaining should be seen in virtually all crypt base cells.
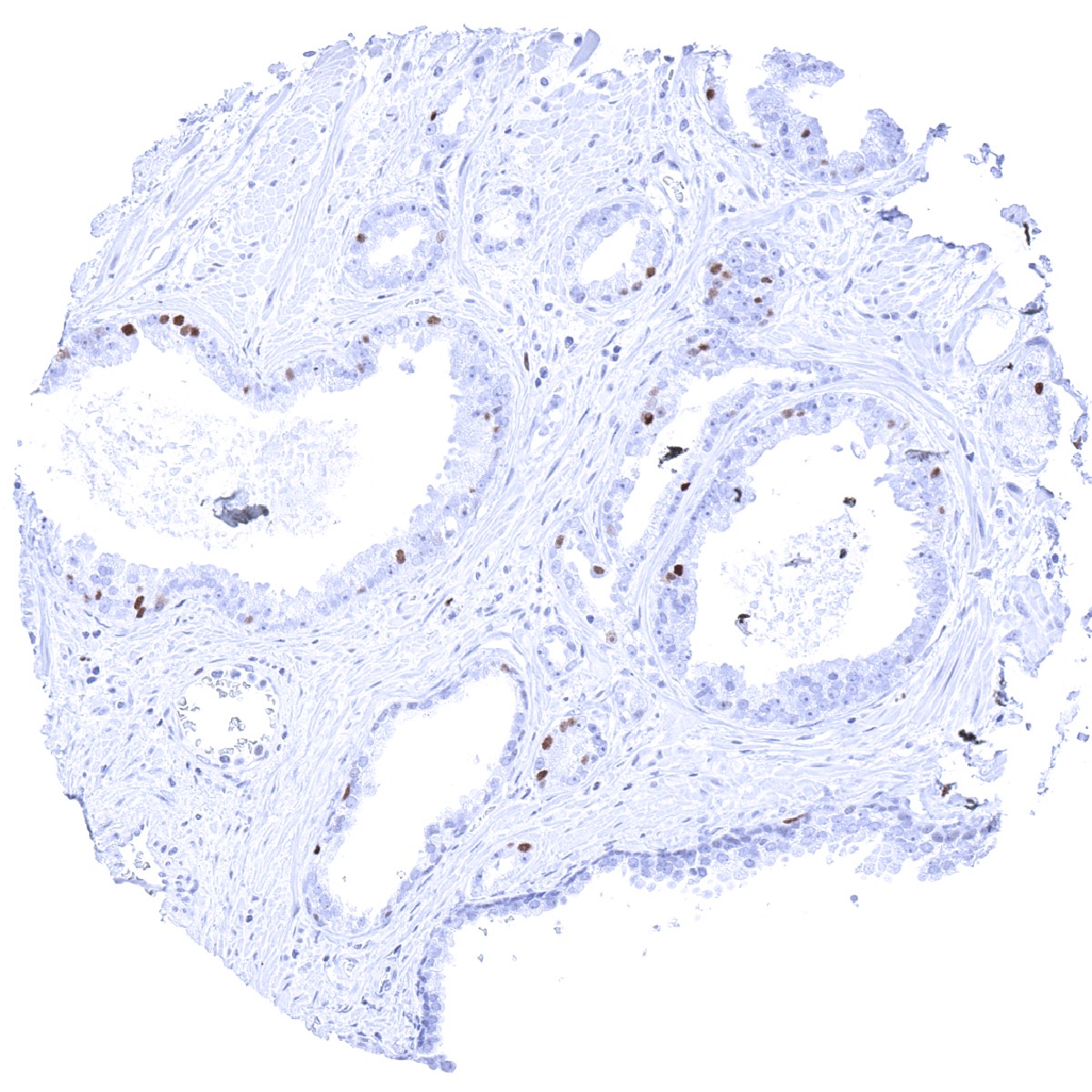
Negative control = Colon: MCM3 immunostaining should be largely absent in surface epithelial cells and in most stroma cells.
Cellular localization = Nuclear
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
MCM3 is a highly sensitive marker for proliferating cells.
Biology Behind
The MCM3 gene is located at 6p12.2 and codes for a 91kDa protein which belongs to the highly conserved mini-chromosome maintenance proteins (MCM) 2-7 that play a key role in genome replication. They form a ring-shaped hexameric protein complex which is essential for the pre-replication complex and may be involved in the formation of replication forks, the recruitment of other DNA replication related proteins, and in maintaining genome integrity. MCM3 is acetylated by the chromatin-associated acetyltransferase MCM3AP. The acetylation of MCM3 inhibits the initiation of DNA replication and cell cycle progression. The MCM proteins are expressed in all cells in the G1, S, G2 and M-phase of the cell cycle but in contrast to the better established proliferation marker Ki-67, MCMs are already expressed in early G1 phase. This results in the detection of more proliferating cells as compared to Ki67 immunohistochemistry which might be advantageous in tumor types with low proliferative activity.
Staining Pattern in Normal Tissues
A nuclear MCM3 immunostaining of variable intensity – mostly strong – occurs in the cell compartments of tissues known to contain proliferating cells. This includes suprabasal and (weaker) basal cell layers of squamous epithelium and urothelium, mucous neck cells of the stomach, crypts of the intestine, as well as a fraction of epithelial cells of the gallbladder, respiratory epithelium and the fallopian tube. In the endometrium, almost all epithelial cells and many stromal cells are MCM3 positive but the rate of positive cells drops in the secretion phase. Among lymphatic organs, a particularly strong MCM3 positivity occurs in most cells of germinal centres and the thymic cortex, and also in scattered individual lymphocytes while a large fraction of lymphocytes shows at least a weak MCM3 staining. Almost all cells of the bone marrow are positive. In the testis, most spermatocytes are positive but mature sperms and probably also spermatogonia are negative. In the placenta, many cells of the cytotrophoblast and a fraction of stroma cells show strong MCM3 staining, while amnion and chorion cells are largely negative. Most granulosa cells and theca interna cells of the ovary exhibit at least a moderate positivity. A variable and often low number of MCM3 positive cells is seen in liver, pancreas, salivary glands, pituitary gland, thyroid, and parathyroid. Tissues and cell types with particularly low rate of MCM3 positive cells include prostatic luminal cells, seminal vesicle, and the endocervix.
A weak to moderate nuclear MCM3 staining can also be seen in most cells of several cell types which are known to not proliferate excessively. This includes heart (weak), skeletal (weak to moderate) and smooth muscle cells (negative to weak), ovarian stroma cells as well as basal cells and other epithelial cells of the epididymis (weak to moderate).
MCM3 is a ubiquitously expressed protein. The findings described above are this consistent with the RNA data described in the Human Protein Atlas (Tissue expression MCM3)
Positive control = Colon: A strong nuclear MCM3 immunostaining should be seen in virtually all crypt base cells.
Negative control = Colon: MCM3 immunostaining should be largely absent in surface epithelial cells and in most stroma cells.
Staining Pattern in Relevant Tumor Types
A nuclear MCM3 immunostaining in a fraction of tumor cells is always seen in cancerous tissues.
The TCGA findings on MCM3 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply MSVA-503M at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-503M 1:300 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-503M 1:300 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Roche – Ventana Discovery ULTRA
Pretreatment for 64 minutes at 100°C (pH 8,4); CM peroxidase blocking for 12 minutes (room temperature), MSVA-503M 1:150 for 20 minutes at 36°C, secondary antibody (anti-mouse HQ) for 12 minutes at 36°C, anti-HQ HRP for 12 minutes at room temperature, DAB at room temperature, hematoxylin II at room temperature for 8 minutes, bluing reagent at room temperature for 4 minutes.
These images depict staining results obtained by the protocol described above. It is of note, that the Ventana machines generally require higher antibody concentrations than other commonly used autostainers because the antibodies are automatically diluted during the procedure. Various other protocols can result in an identical result as shown above. A longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of secondary antibody and or the anti-HQ HRP result in stronger staining, potentially at the cost of more background staining.
Impact of pH
The strongest MCM3 staining by MSVA-503M is obtained at a pH 9,0. However, pH 7,8 results in only a slight reduction of the staining intensity as compared to pH9. We thus consider pH7,8 as optimal for manual staining because of the better tissue preservation at pH7,8 than at pH 9,0.
Potential Research Applications
- Marker for proliferative cells.
- The prognostic role of the percentage of MCM7 positive cells is yet unknown for most tumor entities.
- It is unclear whether MCM7 quantification is equally or better suited than the established Ki67-LI for prognosis assessment.
Evidence for Antibody Specificity in IHC
In principle, there are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. Comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
For proteins such as MCM3 which are expressed in virtually all tissues but restricted to specific cell types and cell compartments, orthogonal validation is not well suited. However, the comparison of MSVA-503M immunostaining data with data from three independent RNA screening studies, including the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project, which are all summarized in the Human Protein Atlas (Tissue expression MCM3) supported the specificity of a weak MCM3 staining observed by MSVA-503M in virtually all cells of few tissue types with little proliferative activity such as heart, skeletal, and smooth muscle cells or ovarian stroma cells.
Comparison of antibodies: A specific staining of the MCM3 antibody MSVA-503M is supported by an identical staining pattern – mainly restricted to proliferating cell types – seen by employing the antibody CAB002162 (used for the analyses within the human protein atlas). The sensitivity of the assay used in the human protein atlas is lower than what is seen for MSVA-503M with the protocol suggested here. Accordingly, staining with CAB002162 is weaker and does not show positivity in cell types for which MSVA-503M immunostaining was weak (heart, skeletal, and smooth muscle cells, ovarian stroma cells).
Indirect confirmation of a low level MCM3 expression in heart, skeletal, and smooth muscle or ovarian stroma cells comes from similiar findings for MCM7 obtained by both our antibody MSVA-507R (link) and the human protein atlas antibody CAB016312.