295,00 € – 995,00 €
Product details
Synonyms = nuclear receptor subfamily 3 group C member 1 , GCCR , GCR , GCRST , GR , GRL
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = HMV304
Positive control = Mature placenta: A strong GR staining should be seen in stroma cells while trophoblastic cells remain negative.
Negative control = Mature placenta: GR staining should be absent in trophoblastic cells.
Cellular localization = Intracellular
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
Glucocorticoid Receptor is a pivotal multifunctional nuclear receptor protein.
Biology Behind
The glucocorticoid receptor (GR, or GCR) is a 777-amino acid multidomain nuclear protein which is coded by the NR3C1 (nuclear receptor subfamily 3, group C, member 1) gene at 5q31 which consists of ten exons (1 to 9β). Together with the other steroid receptors progesterone receptor (PR), androgen receptor (AR), the estrogen receptors (ERα and ERβ), and the mineralocorticoid receptor (MR), GR builds the nuclear receptor (NR) superfamily subgroup 3. GR is the receptor for cortisol and other glucocorticoids. It is expressed in most human cell types and regulates the transcription of thousands of genes involved in metabolism, development, stress and inflammatory responses. In the absence of its ligand, GR is bound to heat shock proteins in the cytoplasm. Ligand binding results in GR dislocation to the nucleus, binding to specific glucocorticoid response elements (GREs), recruitment of context specific transcriptional coregulators, and consequently, either activation or repression of the respective target genes. GR coregulators include a large and diverse group of proteins such as histone modifying enzymes, histone acetyltransferases or deacetylases, and many others. GR and glucocorticoids have a critical impact on a very broad spectrum of physiological processes. For example, GR appears to be an important adaptor for endocrine influence – specifically the stress response – on the brain and may play a critical role in psychological disorders such as depression and post-traumatic stress disorder. GR expression also plays a complex role in tumorigenesis which goes beyond its effects on the immune system. In several tumor types, both an oncogenic and a tumor suppressive function of GR has been found depending on specific tumor conditions.
Staining Pattern in Normal Tissues
Images describing the Glucocorticoid Receptor staining pattern in normal tissues obtained by the antibody HMV304 are shown in our “Normal Tissue Gallery”.
| Brain | Cerebrum | Moderate GR positivity of neuronal cells. |
| Cerebellum | Absence of unequivocal GR staining (perhaps due to overfixation of tissue) | |
| Endocrine Tissues | Thyroid | Moderate to strong GR positivity of follicular epithelial cells. |
| Parathyroid | Moderate to strong GR positivity of epithelial cells. | |
| Adrenal gland | Moderate to strong GR positivity of medullary cells while adrenocortical cells are largely GR negative. | |
| Pituitary gland | Moderate to strong GR positivity of epithelial cells. Weak GR positivity of pituicytes. | |
| Respiratory system | Respiratory epithelium | Moderate to strong GR positivity of respiratory epithelial cells. |
| Lung | Strong GR positivity of pneumocytes. | |
| Gastrointestinal Tract | Salivary glands | Moderate to strong GR positivity of all epithelial cells. |
| Esophagus | Intense GR staining of squamous epithelial cells. Staining intensity decreases sligthly towards the surface. | |
| Stomach | derate GR positivity of superficial epithelial cells. Staining is weakest in gastric glands. | |
| Duodenum | Strong GR positivity of superficial epithelial cells. Staining is somewhat weaker in the crypts.Moderate GR positivity of Brunner gland cells. | |
| Small intestine | Moderate GR positivity of epithelial cells. GR staining is stronger in lymphocytes. | |
| Appendix | Moderate GR positivity of superficial epithelial cells. Epithelial cell staining decreases markedly towards the base of crypts where it can be absent in a fraction of cells. GR staining is markedly stronger in lymphocytes. | |
| Colon | Moderate GR positivity of superficial epithelial cells. Epithelial cell staining decreases markedly towards the base of crypts where it can be absent in a fraction of cells. GR staining is stronger in lymphocytes. | |
| Rectum | Moderate GR positivity of superficial epithelial cells. Epithelial cell staining decreases markedly towards the base of crypts where it can be absent in a fraction of cells. GR staining is markedly stronger in lymphocytes. | |
| Liver | Moderate GR positivity of hepatocytes, ductal cells and of lymphocytes. | |
| Gallbladder | Strong GR positivity of epithelial cells. | |
| Pancreas | Moderate to strong GR positivity of acinar cells while islet cells show a strong staining. | |
| Genitourinary | Kidney | Moderate to strong GR positivity of all epithelial cells. |
| Urothelium | Weak to moderate GR positivity of the basal and suprabasal cell layers of the urohelium. Superficial cell layers are largely GR negative. | |
| Male genital | Prostate | Moderate to strong GR positivity of epithelial cells. |
| Seminal vesicles | Moderate to strong GR positivity of all epithelial cells. | |
| Testis | Intense GR positivity of Sertoli and Leydig cells. Cells of the spermatogenesis do not show significant GR staining. | |
| Epididymis | Moderate to strong GR positivity of all epithelial cells of the caput epididymis. | |
| Female genital | Breast | Moderate to strong GR positivity of myoepithelial and luminal cells.s |
| Uterus, myometrium | Strong GR positivity of myometrial cells. | |
| Uterus, ectocervix | Significant GR staining of squamous epithelial cells. Staining intensity decreases sligthly towards the surface. | |
| Uterus endocervix | Moderate GR positivity of epithelial cells. | |
| Uterus, endometrium | Moderate GR positivity of stroma cells while endometrium cells are largely GR negative. | |
| Fallopian Tube | Strong GR positivity of all epithelial cells. | |
| Ovary | Strong GR positivity of stromal cells.
Moderate to strong GR positivity of corpus luteum cells. Granulosa cells are largely GR negative. |
|
| Placenta early | Strong GR staining of trophoblast and stroma cells. | |
| Placenta mature | Strong GR staining of stroma cells while trophoblast cells are negative. Moderate GR positivity of decidua cells. | |
| Amnion | Strong GR positivity of amnion cells. | |
| Chorion | Strong GR positivity of chorion cells. | |
| Skin | Epidermis | Intense GR positivity of squamous epithelial cells. Staining intensity decreases slightly towards the surface. |
| Sebaceous glands | ||
| Muscle/connective tissue | Heart muscle | Strong GR positivity of heart muscle cells. |
| Skeletal muscle | Strong GR positivity of skeletal muscle cells. | |
| Smooth muscle | Moderate to strong GR positivity of smooth muscle cells. | |
| Vessel walls | Moderate to strong GR positivity of spindle shaped cells in the aortic media. | |
| Fat | Strong GR positivity of fat cells. | |
| Stroma | Moderate to strong GR positivity of all cells. | |
| Endothelium | Moderate GR positivity of endothelial cells. | |
| Bone marrow/lymphoid tissue | Bone marrow | Strong GR positivity of almost all hematopoetic cells. |
| Lymph node | Strong GR positivity of lymphocytic cells. | |
| Spleen | Strong GR positivity of lymphocytic cells. | |
| Thymus | Strong GR positivity of lymphocytic cells. GR staining is only weak in squamous epithelial cells of corpuscles of Hassall‘s. | |
| Tonsil | Strong GR positivity of lymphocytic cells.Intense GR positivity of squamous epithelial cells with somewhat decreasing intensity towards the surface. | |
| Remarks | The vast majority of cell types show strong GR staining. Only few cells are either GR negative or show greatly reduced GR staining. |
In normal tissues, GR is present in every organ and in most individual cell types. These findings are largely consistent with the RNA data described in the Human Protein Atlas (Tissue expression GR). also describing ubiquitous GR expression.
Positive control = Mature placenta: A strong GR staining should be seen in stroma cells while trophoblastic cells remain negative.
Negative control = Mature placenta: GR staining should be absent in trophoblastic cells.
Staining Pattern in Relevant Tumor Types
GR expression occurs in virtually all tumor entities in a large fraction of cases.
The TCGA findings on GR RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
Glucocorticoid Receptor (GR) (HMV304) publication summary
Relevant publication: Tsourlakis et al.: “Glucocorticoid Receptor (GR) Expression in Human Tumors: A Tissue Microarray Study on More than 14,000 Tumors.” Published in Biomedicines. 2025 Jul 9;13(7):1683. . PMID: 40722755
A total of 14,349 tumors were successfully analyzed from 147 different tumor categories by using the following protocol: Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH7,8 Target Retrieval Solution buffer. Apply HMV304 at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent). This protocol was also used for all stainings depicted in our tumor and normal tissue galleries.
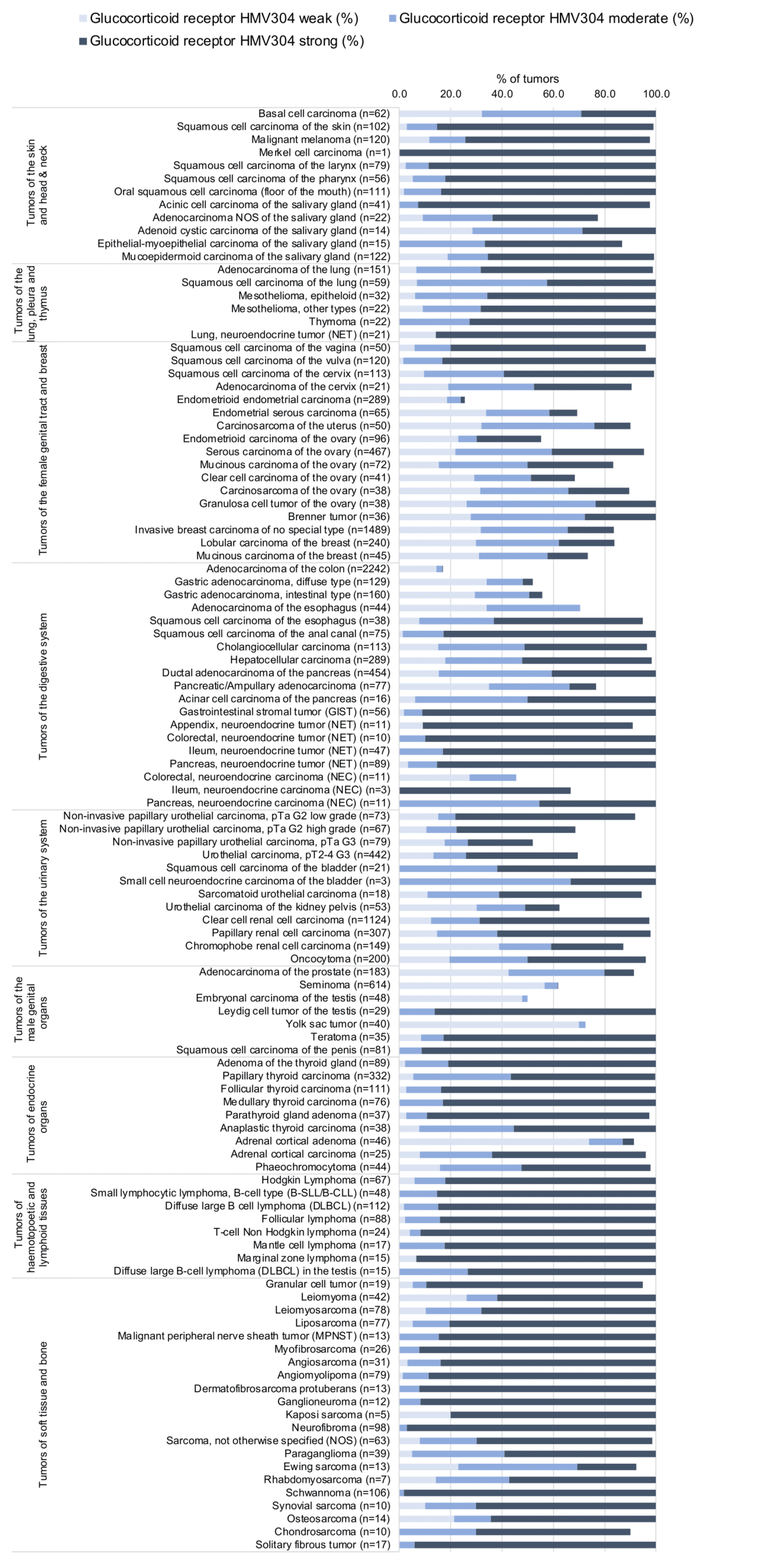
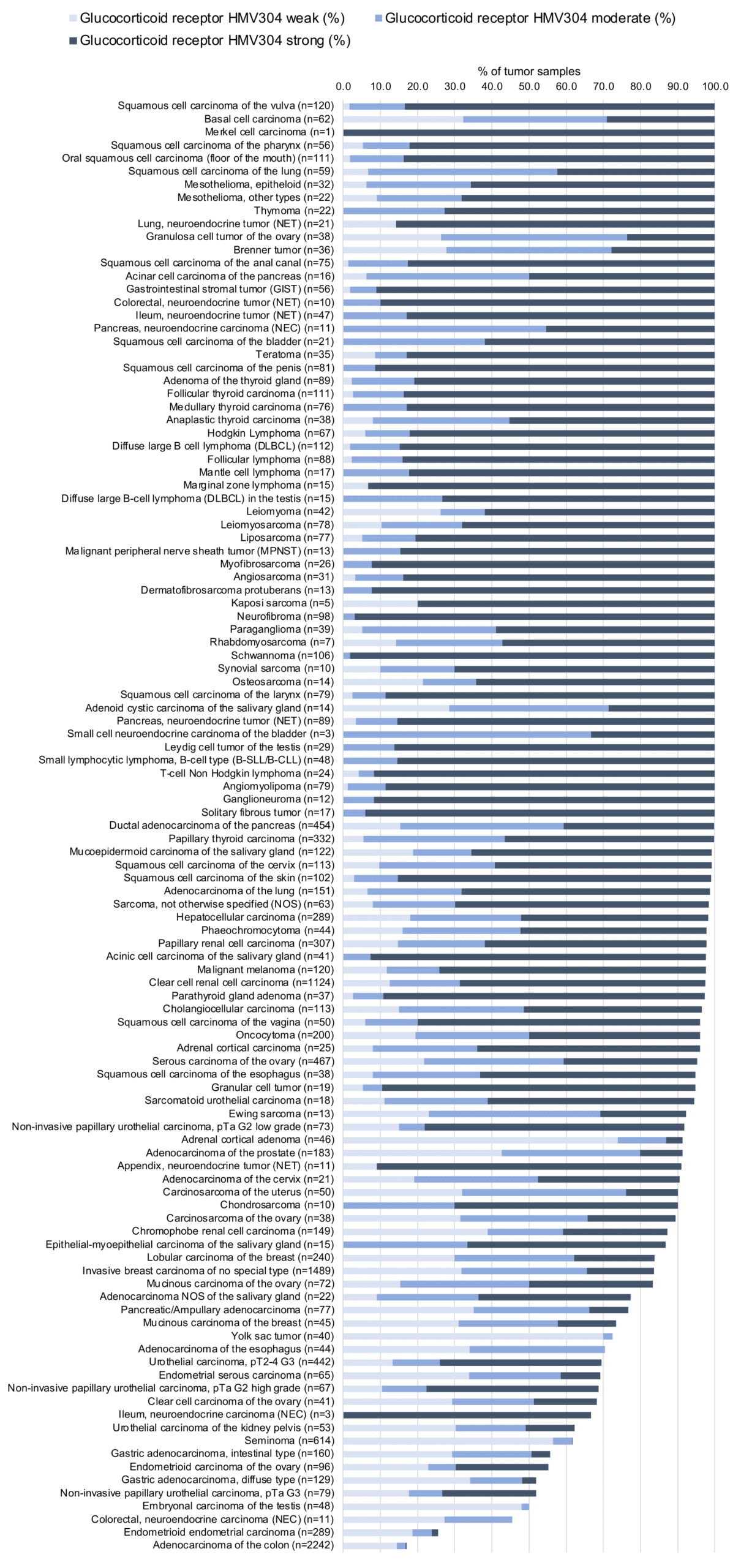
At least one GR positive case was seen in all 147 tumor types and 136 tumor categories included at least one case with strong GR positivity. A total of 77 tumor entities showed GR positivity at variable intensity in all analyzed cases while 43 tumor entities showed GR positivity in 80–99.9% of cases, and 21 entities showed GR positivity in 50-80% of cases. Only six tumor types had less than 50% GR-positive cases. These included adenomas with low (32.5%) – and high-grade dysplasia (21.7%), adenocarcinomas (17%) and neuroendocrine carcinomas (45.5%) of the colorectum, as well as endometrioid endometrial carcinomas (25.6%) and rhabdoid tumors (25%). The distribution of positive and negative staining results is shown in an “organ-systematic” and in a “ranking order” figure below (images based on data from Tsourlakis et al.). Results on possible associations with histopathological and clinical parameters of tumor aggressiveness are also summarized below (table based on data from Tsourlakis et al.).
Authors conclusions on diagnostic utility with respect to the distinction of reactive vs. neoplastic (Tsourlakis et al.):
- Not applicable
Authors conclusions on diagnostic utility with respect to the distinction of benign versus malignant (Tsourlakis et al.):
- Because 88% of 25 adrenocortical carcinomas showed moderate to strong GR positivity while only 15,5% of 47 adrenocortical adenomas showed moderate to strong GR positivity, GR IHC may serve as a marker of malignancy in adrenocortical neoplasms.
Authors conclusions on diagnostic utility with respect to the distinction of different tumor entities (Tsourlakis et al.):
- Based on the very low frequency of colorectal adenocarcinomas with moderate (2.4%) and strong (0.2%) GR positivity, a significant GR positivity by IHC would argue against a colorectal origin of an adenocarcinoma metastasis. Other adenocarcinomas, such as those from the pancreas (84.3%), the gallbladder (77.1%), the stomach (24.4%), the esophagus (36.4%), the lung (92.1%), or the prostate (56.7%) had markedly higher rates of moderate-to-strong GR positivity.
Authors conclusions on prognostic role of GR expression (Tsourlakis et al.):
- low GR expression is associated with advanced pT stage (p=0,0006) and absence of ER expression (p=0,0126) in breast cancer of no special type.
- low GR expression is associated with advanced pT stage (p<0,0001) and absence of high grade (p<0,01) in clear cell renal cell carcinoma.
- low GR expression is associated with advanced pT stage (p=0,0153) in papillary cell renal cell carcinoma.
- low GR expression is associated with high grade in non-invasive (p<0,0001) and with nodal metastasis (p=0,0051) in invasive urothelial carcinoma.
- low GR expression is associated with MSI in gastric adenocarcinoma (p=0,0051).
Data from the publication: “Glucocorticoid Receptor (GR) Expression in Human Tumors: A Tissue Microarray Study on More than 14,000 Tumors.” Published by Tsourlakis et al. in Biomedicines. 2025 Jul 9;13(7):1683. . PMID: 40722755 – Summarized in own graphics:
1. Glucocorticoid Receptor staining in tumors “organ-specific” with antibody HMV304
2. Glucocorticoid Receptor staining in tumors “ranking order” by positivity with antibody HMV304
3. Table Clinico-pathological associations described by Tsourlakis et al. (p-value)
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply HMV304 at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Potential Research Applications
The diagnostic, prognostic, and predictive relevance of GR expression in tumors and in preneoplastic disease is unknown.
Evidence for Antibody Specificity in IHC
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. Comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
Orthogonal validation: For proteins such as GR which are expressed in all organs, orthogonal validation is not suited.
Comparison of antibodies: True expression of GR in all cell types found to be GR positive by HMV304 is corroborated by identical stainings obtained by another commercially available independent antibody (termed “validation antibody”). Both antibodies showed a significant reduction or loss of GR expression in the same cell types (upper cell layers of non-keratinizing squamous epithelium and of the urothelium, cells of the spermiogenesis, granulosa cells of the ovary, adrenocortical cells, and trophoblastic cells of the mature (but not of the first trimenon) placenta).