Product details
Synonyms = CCNE1; Cyclin E1; G1/S-specific cyclin-E1, G1/S-specific cyclin-E1
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-898R
Positive control = Placenta: A strong Cyclin E1 staining should be seen in most cytotrophoblast cells.
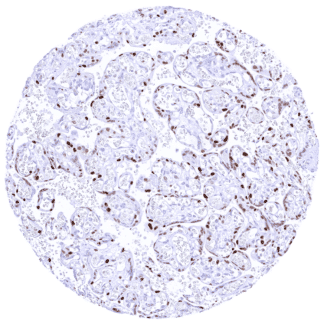
Negative control = Normal kidney: Cyclin E1 staining should be largely absent in tubular and glomerular cells.
Cellular localization = Nucleus
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:50 – 1:100
Intended Use = Research Use Only
Relevance of Antibody
Cyclin E1 is a critical protein for cell-cycle control.
Biology Behind
Cyclin E1 is a 47 kDa protein coded by the CCNE1 gene at 19q12. It has 47% homology and largely overlapping functions with Cyclin E2. Similar to all cyclin family members, cyclin E1 binds to cyclin-dependent kinases, in particular CDK2. The cyclin E/CDK2 complex regulates various downstream proteins by phosphorylation and plays a critical role in the G1 phase and in the G1-S phase transition of the cell cycle. For example, cyclin E/CDK2 phosphorylates retinoblastoma protein (Rb) to promote G1 progression. As a result, hyper-phosphorylated Rb will no longer interact with E2F transcriptional factor and release it to promote expression of further genes needed for entering S phase. Other important cell cycle regulatory proteins phosphorylated by cyclin E/CDK2 include p27, p21, CBP/p300, p220(NPAT), and E2F-5. Independent of its role in cell cycle progression, cyclin E/CDK2 plays a role in the centrosome cycle. Cyclin E1 has been described to be overexpressed in cancers of various types. Cyclin E1 amplification, one of the mechanisms for overexpression, has been suspected to lead to synthetic lethality in combination with PKMYT1 kinase inhibition.
Staining Pattern in Normal Tissues
Images describing the MCM2 staining pattern in normal tissues obtained by the antibody MSVA-898R are shown in our “Normal Tissue Gallery”.
| Brain | Cerebrum | Negative. |
| Cerebellum | Negative. | |
| Endocrine Tissues | Thyroid | Negative. |
| Parathyroid | Weak to moderate Cyclin E1 staining of epithelial cells. | |
| Adrenal gland | Weak to moderate Cyclin E1 staining of adrenocortical cells. | |
| Pituitary gland | Negative. | |
| Respiratory system | Respiratory epithelium | Negative. |
| Lung | Negative. | |
| Gastrointestinal Tract | Salivary glands | Faint Cyclin E1 staining of few epithelial, mainly ductal cells. |
| Esophagus | Weak to strong nuclear Cyclin E1 staining of squamous epithelial cells in the upper half of the surface epithelium. The staining intensity gradually increases towards the surface. | |
| Stomach | Weak to moderate Cyclin E1 staining of a subset of cells (predominantly neck cells). | |
| Duodenum | Weak to moderate Cyclin E1 staining of a subset of crypt cells. | |
| Small intestine | Weak to moderate Cyclin E1 staining of a subset of crypt cells. | |
| Appendix | Weak to moderate Cyclin E1 staining of a large subset of crypt cells. Weak to moderate Cyclin E1 staining of a subset of muscle cells. Strong Cyclin E1 positivity of intramural ganglion cells. | |
| Colon | Weak to moderate Cyclin E1 staining of a large subset of crypt cells. Weak to moderate Cyclin E1 staining of a subset of muscle cells. | |
| Rectum | Weak to moderate Cyclin E1 staining of a large subset of crypt cells. Weak to moderate Cyclin E1 staining of a subset of muscle cells. | |
| Liver | Negative. | |
| Gallbladder | A weak Cyclin E1 staining of few epithelial cells can occur. | |
| Pancreas | Negative. | |
| Genitourinary | Kidney | A weak nuclear Cyclin E1 staining of a subset of collecting duct cells can be seen in individual samples. |
| Urothelium | Weak to strong Cyclin E1 staining of urothelial cells is limited to the top cell layers of the urothelium. The staining intensity increases towards the surface. | |
| Male genital | Prostate | Negative. |
| Seminal vesicles | Negative. | |
| Testis | Weak to moderate Cyclin E1 staining of spermatocytes. | |
| Epididymis | Negative. | |
| Female genital | Breast | Faint Cyclin E1 staining of few epithelial cells. |
| Uterus, myometrium | Negative. | |
| Uterus, ectocervix | Faint Cyclin E1 staining of squamous epithelial cells of the upper 2/3 of the epithelial cell layer. | |
| Uterus endocervix | Negative. | |
| Uterus, endometrium | Strong Cyclin E1 staining of a large fraction of epithelial cells (especially during the proliferation phase) while staining is weak in a fraction of stroma cells. During pregnancy, a strong Cyclin E1 staining can be seen in decidua cells. | |
| Fallopian Tube | A weak Cyclin E1 staining can occur in few epithelial cells. | |
| Ovary | Weak Cyclin E1 staining of corpus luteum cells. | |
| Placenta early | Strong Cyclin E1 staining of most cytotrophoblast cells while stroma cells stain less intensively. | |
| Placenta mature | Strong Cyclin E1 staining of many/most cytotrophoblast cells. | |
| Amnion | Weak to strong Cyclin E1 staining can be seen in a fraction of amnion cells. | |
| Chorion | Strong Cyclin E1 staining of most chorion cells. | |
| Skin | Epidermis | Weak to strong Cyclin E1 staining of squamous epithelial cells of the upper half of the epidermis. The staining intensity gradually increases towards the surface. |
| Sebaceous glands | ||
| Muscle/connective tissue | Heart muscle | Very faint Cyclin E1 staining may occur in few cells. |
| Skeletal muscle | Very faint Cyclin E1 staining may occur in few cells. | |
| Smooth muscle | Faint to moderate Cyclin E1 staining may occur in few cells. | |
| Vessel walls | Negative. | |
| Fat | Negative. | |
| Stroma | Cyclin E1 staining of variable intensity can occur in a fraction of stroma cells (inflammatory, myofibroblasts, others) | |
| Endothelium | Negative. | |
| Bone marrow/ lymphoid tissue | Bone marrow | Weak to strong Cyclin E1 staining of a subset of hematopetic cells. |
| Lymph node | Weak to moderate Cyclin E1 staining of a fraction of lymphocytic cells, especially in germinal centres. | |
| Spleen | Weak Cyclin E1 staining of only a small fraction of cells. | |
| Thymus | Strong Cyclin E1 staining of some regularly distributed cells (thymic epithelial cells?). Weak to moderate Cyclin E1 staining of some lymphocytic cells. | |
| Tonsil | Weak to moderate Cyclin E1 staining of a fraction of lymphocytic cells, especially in germinal centres. Strong Cyclin E1 staining of most squamous epithelial cells in the upper half of the surface epithelium. | |
| Remarks | A Cyclin E1 staining of variable intensity (often only very faint) can occur in practically all tissues, especially in case of inflammation and reparation. |
Cyclin E1 staining was always nuclear. A particularly strong Cyclin E1 positivity in a large fraction of cells occurred in chorion cells, cytotrophoblast cells, and decidua cells of the placenta, epithelial cells of proliferating endometrium, superficial cell layers of the urothelium and squamous epithelium (especially skin and tonsil). A weak to moderate staining was also seen in epithelial cells of the adrenal cortex and of the parathyroid gland. A faint to weak to moderate Cyclin E1 staining could further be observed in virtually every tissue in cell types/tissue areas with proliferative activity. These for example included tissues with inflammation or reparation, crypt cells in the intestine, neck cells of the stomach mucosa, lymphocytic cells in germinal centres or the thymus, spermatocytes of the testis, amnion cells, and hematopetic cells of the bone marrow. A particular strong Cyclin E1 occurred in chorion cells, cytotrophoblast cells, and decidua cells of the placenta, epithelial cells of proliferating endometrium, superficial cell layers of the urothelium and squamous epithelium (especially skin and tonsil). Tissues with particularly low or absent Cyclin E1 expression included, skeletal and heart muscle, the brain, pituitary gland, thyroid, prostate, seminal vesicle, epididymis, kidney, breast, endocervix, fallopian tube, lung, respiratory epithelium.
The findings described above are thus consistent with the RNA data described in the Human Protein Atlas (Tissue expression Cyclin E1). The particularly high RNA expression levels in the placenta, the bone marrow, the testis, and the adrenal gland are consistent with IHC data obtained by MSVA-898R.
Positive control = Placenta: A strong Cyclin E1 staining should be seen in most cytotrophoblast cells.
Negative control = Normal kidney: Cyclin E1 staining should be largely absent in tubular and glomerular cells.
Staining Pattern in Relevant Tumor Types
Cyclin E1 can be overexpressed in a broad range of different tumor entities including for example tumors of the breast, cervix, endometrium, ovary, esophagus, stomach, colon, gallbladder, liver, prostate, testis, urinary bladder, head and neck, lung, bone, skin, thyroid, and the lymphatic system.
The TCGA findings on Cyclin E1 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply MSVA-898R at a dilution of 1:75 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Potential Research Applications
- The diagnostic and prognostic relevance of Cyclin E1 expression in tumors and in preneoplastic disease needs to be further investigated.
- The prevalence of Cyclin E1 overexpression in different tumor entities needs to be determined.
- The role of Cyclin E1 overexpression as a trigger for synthetic lethality in combination with PKMYT1 kinase inhibition must be further evaluated.
Evidence for Antibody Specificity in IHC
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. Comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
Orthogonal validation: For the antibody MSVA-898R specificity is supported by the good concordance of the immunostaining data with data from three independent RNA screening studies, including the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project, which are all summarized in the Human Protein Atlas (Tissue expression Cyclin E1). The particularly high RNA expression levels in the placenta, the bone marrow, the testis, and the adrenal gland are consistent with IHC data obtained by MSVA-898R as these tissues either contained cells with a very strong Cyclin E1 staining or a high density of cells with a weak to moderate Cyclin E1 staining. Orthogonal validation is, however, not optimal for validating antibodies for proteins that are ubiquitously expressed cell-cycle related proteins such as Cyclin E1.
Comparison of antibodies: True expression of Cyclin E1 in all cell types found Cyclin E1 positive by MSVA-898R is corroborated by identical stainings obtained by another commercially available independent antibody (termed “validation antibody”). The significant additional cytoplasmic staining obtained by the validation antibody in numerous cell types in various tissues demonstrates “independence” of the validation antibody.