145,00 € – 995,00 €
Product details
Synonyms = B220, CD45R, GP180, Leukocyte common antigen (LCA), Loc, Ly-5, Lyt-4, Protein tyrosine phosphatase receptor type C (PTPRC), Receptor-type tyrosine-protein phosphatase C, T200 glycoprotein
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-045R
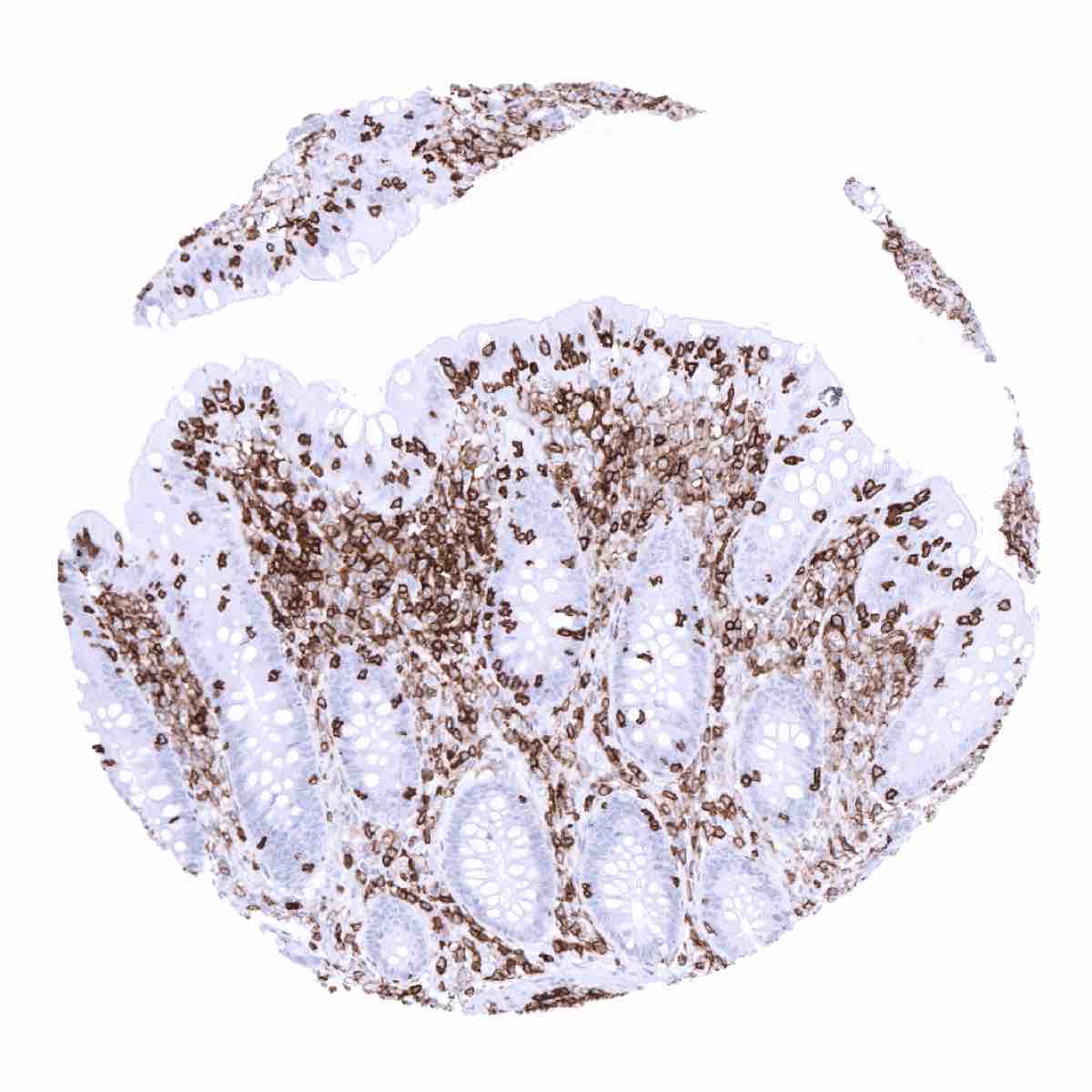
Positive control = Tonsil: All lymphocytes and histiocytes must show a strong membranous CD45 staining.
Negative control = Tonsil: Squamous epithelial cells must be completely CD45 negative.
Cellular localization = Cell Surface & Cytoplasmic
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
CD45 is expressed on hematolymphoid cells.
Biology Behind
CD45 is a type I transmembrane protein that is present in various isoforms on all differentiated hematopoietic cells except erythrocytes and plasma cells. It is thus also called leukocyte common antigen (LCA). The gene is located at 1q31.3-q32.1 and contains 34 exons coding for an unusually large protein with extracellular and cytoplasmic domains. Exons 4, 5, and 6 (corresponding to protein regions A, B, and C) are alternatively spliced to generate up to eight different protein products featuring combinations of zero, one, two, or all three of these exons. All CD45 isoforms are essential regulators of T- and B-cell antigen receptor signaling. They function through either direct interaction with components of the antigen receptor complexes via its extracellular domain or by activating various Src family kinases required for the antigen receptor signaling via its cytoplasmic domain. Isoforms lacking at least one of the protein regions A, B, or C are termed CD45R (“restricted”).
Staining Pattern in Normal Tissues
CD45 is exclusively expressed on membranes of hematolymphoid cells. Almost all hematolymphoid cell types, including granulocytes, precursor cells, mature B- and T-lymphocytes, monocytes/histiocytes, interdigitating reticulum cells, and follicular dendritic cells show variable levels of CD45 immunostaining. CD45 is strongly expressed on lymphocytes while monocytes/histiocytes show a somewhat weaker staining. CD45 expression is lost in maturing erythrocytes, megakaryocytes and plasma cells.
These findings are largely comparable to the RNA and protein data described in the Human Protein Atlas (Tissue expression CD45)
Positive control = Tonsil: All lymphocytes and histiocytes must show a strong membranous CD45 staining.
Negative control = Tonsil: Squamous epithelial cells must be completely CD45 negative.
Staining Pattern in Relevant Tumor Types
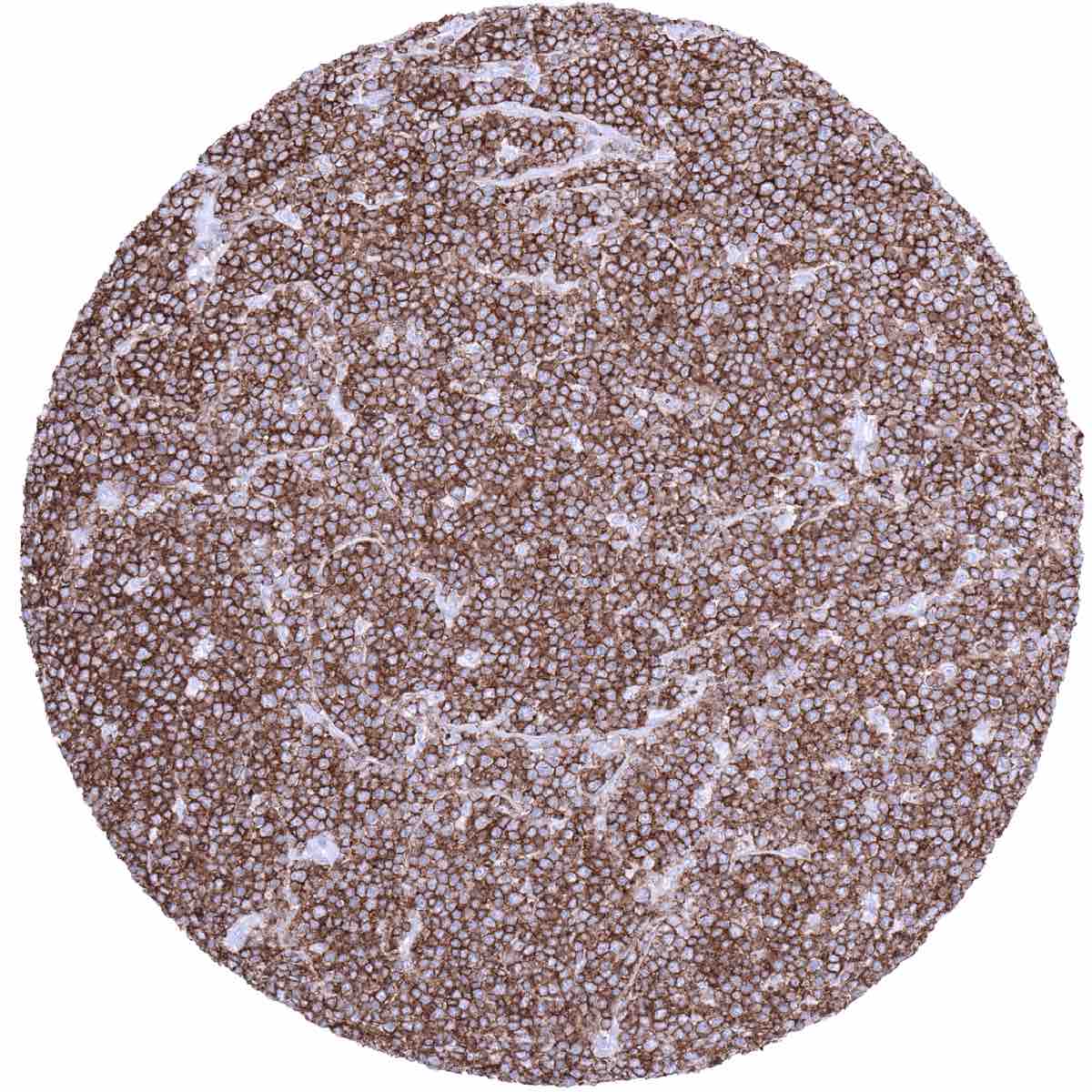
A positive CD45 immunostaining occurs in the vast majority of leukemias, malignant lymphomas, mast cell neoplasms as well as of histiocytic and dendritic cell neoplasms. More than 90% of malignant lymphomas express detectable CD45 levels. Among lymphomas, the CD45 positivity rate is lowest in precursor B-cell neoplasms, large cell anaplastic lymphomas, and plasmacytic neoplasms (positive in about 10%). In Hodgkin’s lymphoma, the L&H cells are always positive in the LP-type, but Reed-Sternberg are negative or only show faint cytoplasmic staining cells in classic Hodgkin’s lymphoma.
The TCGA findings on CD45 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein. Accordingly, multiple different protocols can generate identical staining results.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7.8 Target Retrieveal Solution buffer. Apply MSVA-045R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-045R 1:150 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-045R 1:150 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Roche – Ventana Discovery ULTRA
Pretreatment for 64 minutes at 100°C (pH 8,4); CM peroxidase blocking for 12 minutes (room temperature), MSVA-045R 1:150 for 20 minutes at 36°C, secondary antibody (anti-mouse HQ) for 12 minutes at 36°C, anti-HQ HRP for 12 minutes at room temperature, DAB at room temperature, hematoxylin II at room temperature for 8 minutes, bluing reagent at room temperature for 4 minutes.
These images depict staining results obtained by the protocol described above. It is of note, that the Ventana machines generally require higher antibody concentrations than other commonly used autostainers because the antibodies are automatically diluted during the procedure. Various other protocols can result in an identical result as shown above. A longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of secondary antibody and or the anti-HQ HRP result in stronger staining, potentially at the cost of more background staining.
Potential Research Applications
- The prevalence of CD45 expression in hematological and non-hematological neoplasms should be further investigated.
- CD45 is an important component of multicolor immunohistochemistry assays analyzing the role of subsets of hematolymphoid cells.
Evidence for Antibody Specificity in IHC
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. Comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
Orthogonal validation: Comparison with RNA expression data is not well suited to validate immunohistochemical stainings of cell types such as hematolymphoid cells which occur in virtually all organs. Nevertheless, in agreement with data from three independent RNA screening studies, including the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project, which are all summarized in the Human Protein Atlas (Tissue expression CD45), CD45 immunostaining with MSVA-045R revealed the highest fractions of positive cells in the bone marrow and in lymphoid tissues.
Comparison of antibodies: The validity of CD45 immunostaining is strongly supported by a similar distribution of positive cells found by the independent antibody CAB000052 for which staining images are provided in the human protein atlas.