Product details
Synonyms = B-lymphocyte cell adhesion molecule (BL-CAM); B-cell receptor CD22; CD22; Lectin 2; Lyb8; Sialic acid-binding Ig-like lectin 2 (Siglec-2); SIGLEC2; T-cell surface antigen Leu-14
Antibody type = Recombinant Rabbit monoclonal / IgG
Clone = MSVA-022R
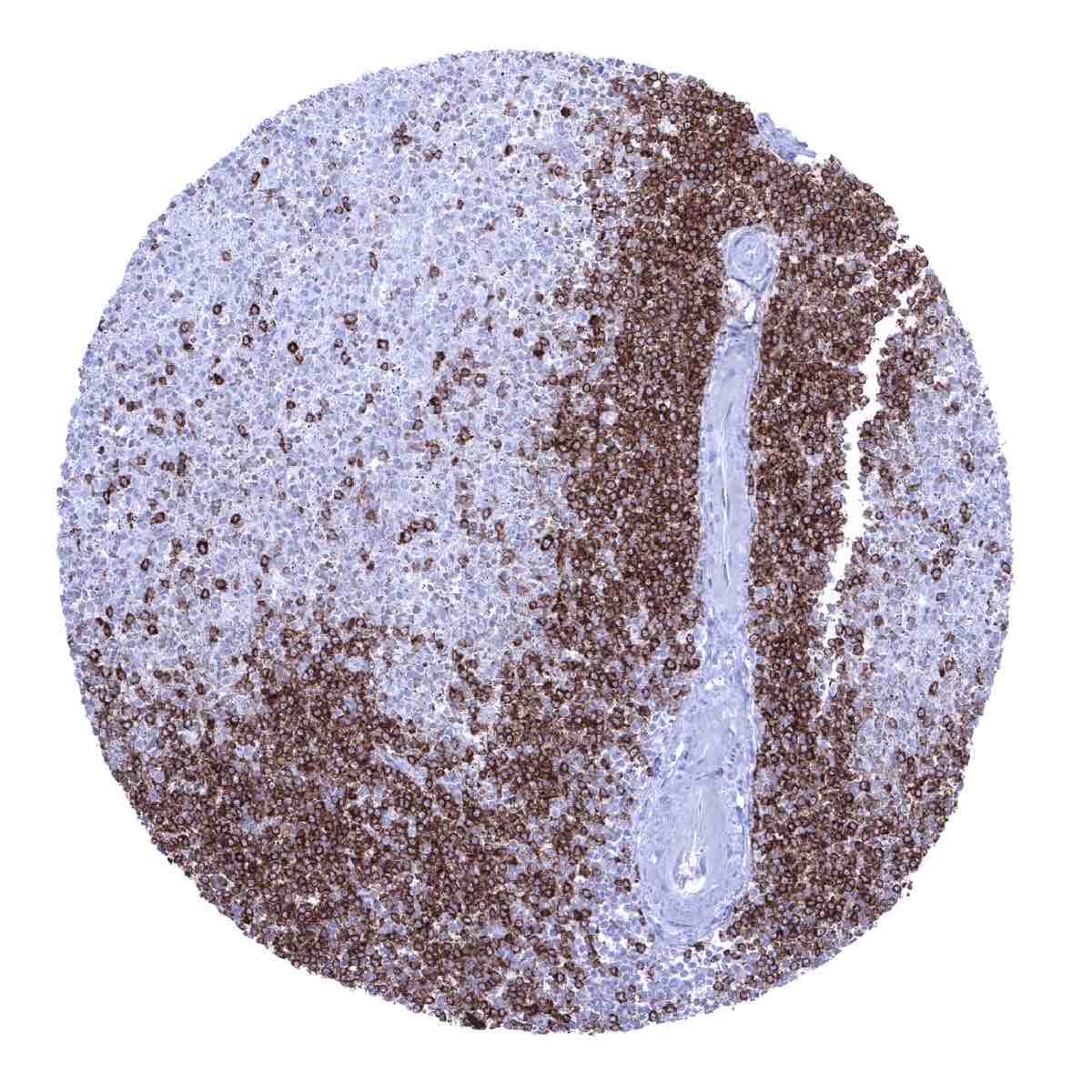
Positive control = Tonsil or appendix: A strong, predominantly membranous CD22 staining of the germinal centre and mantle zone B-cells as well as of few interfollicular B-cells should be seen.
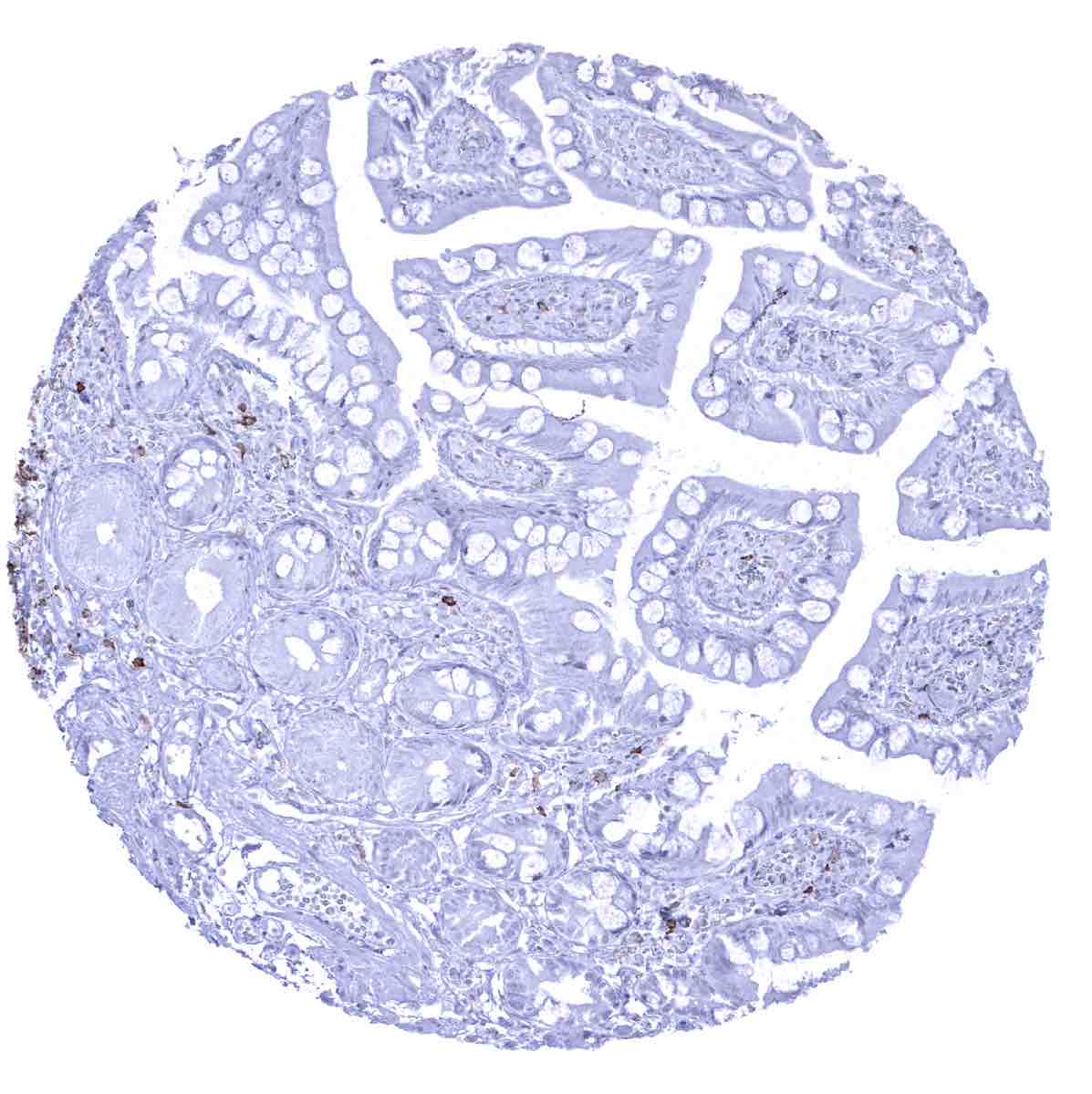
Negative control = Tonsil or appendix: A large fraction of the interfollicular lymphocytes and all epithelial cells must not show any CD22 staining.
Cellular localization = Cell Surface
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
CD22 is expressed on early B lymphocytes.
Biology Behind
CD22 is a cell surface protein coded by the CD22 gene located at 19q13.12. CD22 belongs to the SIGLEC family of lectins and is a member of the immunoglobulin superfamily. It is a transmembrane protein, which binds sialic acid with an immunoglobulin (Ig) domain. CD22 plays a role in the regulation of expression of surface IgM on B cells and functions as an inhibitory receptor for B cell receptor signaling. As such, CD22 prevents the excessive activation of the B-cell immune reaction and the development of autoimmune diseases. CD22 is the target of antibody-drug conjugates (inotuzumab ozogamicin, moxetumomab pasudotox) which have been approved for treating hairy cell leukemia and acute lymphoblastic leukemia.
Staining Pattern in Normal Tissues
CD22 is expressed on the vast majority of B-lymphocytes. CD22 positive cells are thus preferably seen in lymphatic organs especially in germinal follicles and mantle zones but can be found in virtually every organ. Cytoplasmic CD22 is expressed at the earliest stages of B-cell differentiation, prior to the expression of CD20. CD22 expression is lost in plasma cells.
These findings are largely consistent with the RNA and protein data described in the Human Protein Atlas (Tissue expression CD22)
Positive control = Tonsil or appendix: A strong, predominantly membranous CD22 staining of the germinal centre and mantle zone B-cells as well as of few interfollicular B-cells should be seen.
Negative control = Tonsil or appendix: A large fraction of the interfollicular lymphocytes and all epithelial cells must not show any CD22 staining.
Staining Pattern in Relevant Tumor Types
Most immature and mature B-cell neoplasms express CD22 at a variable extent. CD22 immunostaining can be particularly strong in hairy cell leukemia and prolymphocytic leukemia. T cells and their malignant counterparts, except for rare cases, do not express CD22.
The TCGA findings on CD22 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply MSVA-022R at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-022R 1:150 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 40 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-022R 1:150 for 20 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Roche – Ventana Discovery ULTRA
Pretreatment for 64 minutes at 100°C (pH 8,4); CM peroxidase blocking for 12 minutes (room temperature), MSVA-022R 1:150 for 20 minutes at 36°C, secondary antibody (anti-mouse HQ) for 12 minutes at 36°C, anti-HQ HRP for 12 minutes at room temperature, DAB at room temperature, hematoxylin II at room temperature for 8 minutes, bluing reagent at room temperature for 4 minutes.
These images depict staining results obtained by the protocol described above. It is of note, that the Ventana machines generally require higher antibody concentrations than other commonly used autostainers because the antibodies are automatically diluted during the procedure. Various other protocols can result in an identical result as shown above. A longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of secondary antibody and or the anti-HQ HRP result in stronger staining, potentially at the cost of more background staining.
Potential Research Applications
- The prevalence of CD22 immunostaining in hairy cell leukemia and other hematological as well as non-hematological neoplasms should be further evaluated.
- The potential of CD22 as a therapeutic target is under investigation.
Evidence for Antibody Specificity in IHC
There are two ways, how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. Comparison with a second independent method for measuring target expression across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
As a standard orthogonal validation process for MSVA antibodies, RNA data summarized in the Human Protein Atlas (Tissue expression CD22) are used for comparison. However, this procedure has only limited validity for proteins that are expressed in significant subsets of lymphocytes because these cells occur in virtually all organs. Nevertheless, in agreement with MSVA-022R staining properties, CD22 RNA is preferentially expressed in lymphatic issues.
The validation of MSVA-022R is therefore largely based on a comparison with a second independent, commercially available CD22 antibody (termed “Validation-antibody”). Although staining was somewhat weaker for the validation antibody, this comparison of antibodies revealed identical staining patterns for MSVA-022R and the “Validation-antibody” with identical subsets of lymphocytes staining. Examples of stainings for both antibodies are shown below (tonsil, lymph node, spleen, thymus, appendix, urinary bladder).
It is also of note, that the staining pattern of MSVA-022R (and the validation antibody) match the expectations as a CD22 staining is preferentially seen in lymphocytes that are located in areas consistent with a B-cell origin (follicular/parafollicular).
Antibody Comparison: MSVA-022R vs another commercially available CD22 antibody called “Validation Antibody”