295,00 € – 995,00 €
Product details
Synonyms = BILL-cadherin; Cadherin-17; CDH17; HPT-1 cadherin; human intestinal peptide-associated transporter HPT-1; human peptide transporter 1 (HPT-1); Intestinal peptide-associated transporter HPT-1; LI-cadherin (liver-intestine); Liver Cadherin; Liver-intestine cadherin
Antibody type = Mouse monoclonal / Mouse IgG2b, kappa
Clone = MSVA-517M
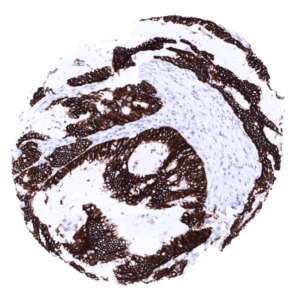
Positive control = Colon: A strong CDH17 staining should be seen in all epithelial cells.
Negative control = Colon: Non-epithelial cells should not show any CDH17 immunostaining.
Cellular localization = Cell Surface and Cytoplasmic
Reactivity = Human
Application = Immunohistochemistry
Dilution = 1:100 – 1:200
Intended Use = Research Use Only
Relevance of Antibody
Biology Behind
Cadherin-17 (CDH17) is a 92,2 kDa protein coded by the CDH17 gene on chromosome 8q22.1. As other members of the cadherin superfamily, CDH17 is a calcium-dependent, membrane-associated glycoprotein. CDH17 acts as an adhesion protein and has a role as a proton-dependent peptide transporter. CDH17 is involved in absorption of various peptide-based drugs. CDH17 was also suggested to be relevant for the morphological organization of liver and intestine. In cancer, aberrant CDH17 expression was found to be linked to unfavorable tumor features in several cancer entities.
Staining Pattern in Normal Tissues
CDH17 staining pattern in Normal Tissues with antibody MSVA-517M (images are shown in our “Normal Tissue Gallery”)
| Brain | Cerebrum | Negative. |
| Cerebellum | Negative. | |
| Endocrine Tissues | Thyroid | Negative. |
| Parathyroid | Negative. | |
| Adrenal gland | Negative. | |
| Pituitary gland | Negative. | |
| Respiratory system | Respiratory epithelium | Negative. |
| Lung | Negative. | |
| Gastrointestinal Tract | Salivary glands | Negative. |
| Esophagus | Negative. | |
| Stomach | Negative. | |
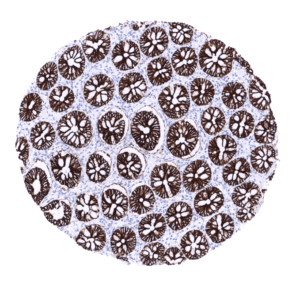
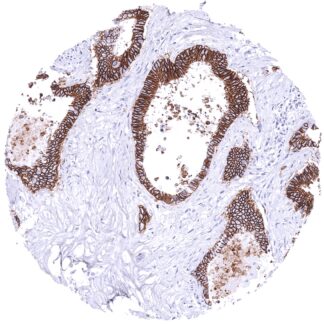
| Duodenum | Strong membranous, predominantly basolateral CDH17 staining of all epithelial cells. | |
| Small intestine | Strong membranous, predominantly basolateral CDH17 staining of all epithelial cells. | |
| Appendix | Strong membranous, predominantly basolateral CDH17 staining of all epithelial cells. | |
| Colon | Strong membranous, predominantly basolateral CDH17 staining of all epithelial cells. | |
| Rectum | Strong membranous, predominantly basolateral CDH17 staining of all epithelial cells. | |
| Liver | Negative. | |
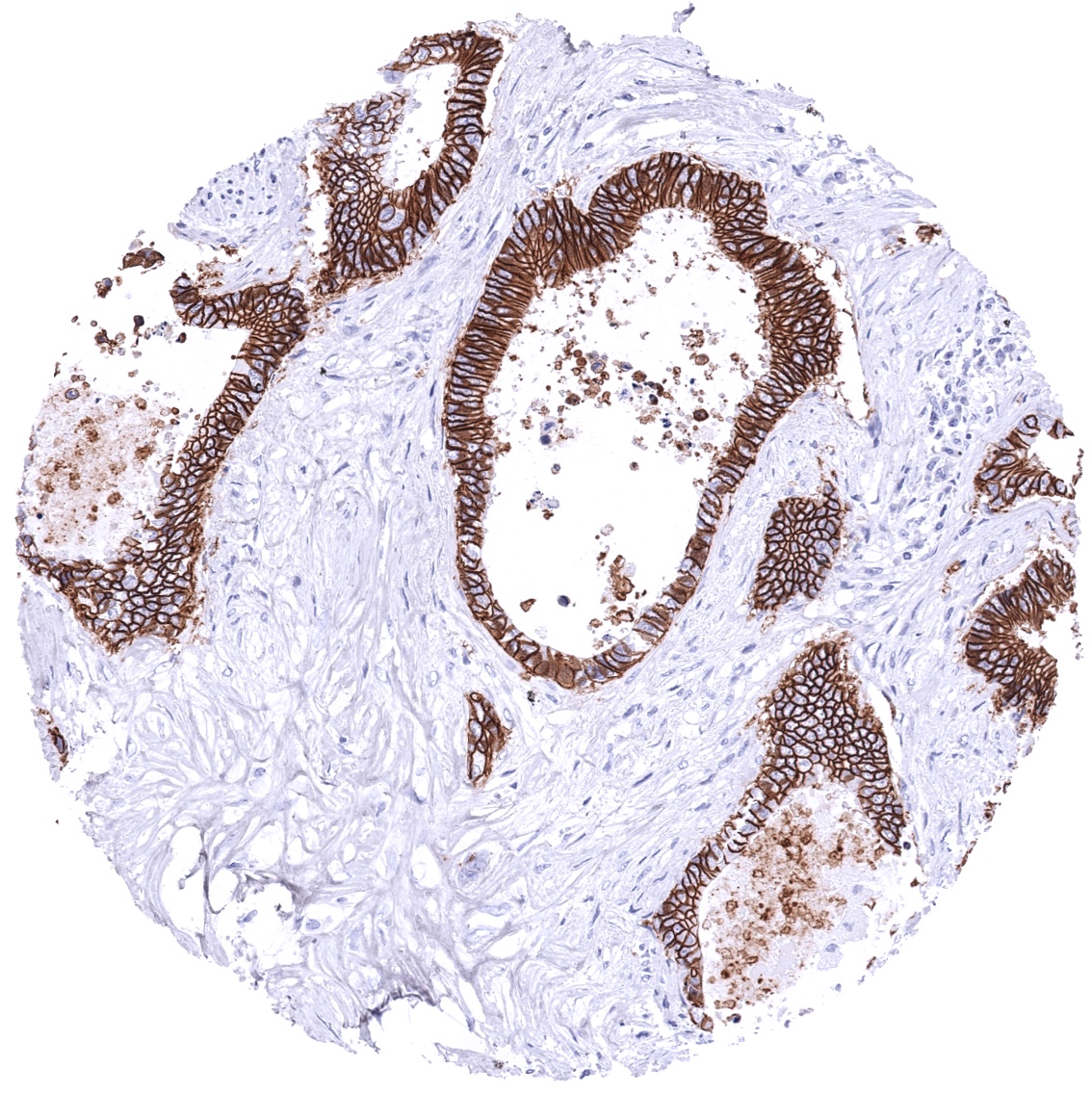
| Gallbladder | A focal CDH17 staining of variable intensity is often seen in epithelial cells. | |
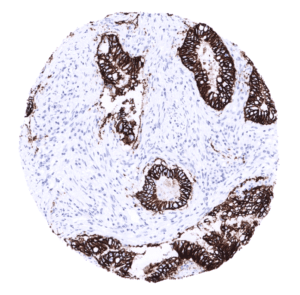
| Pancreas | Strong membranous, predominantly basolateral CDH17 staining of epithelial cells from excretory ducts. Intesity and fraction of positive cells is larger in large than in small ducts. | |
| Genitourinary | Kidney | Negative. |
| Urothelium | Negative. | |
| Male genital | Prostate | Negative. |
| Seminal vesicles | Negative. | |
| Testis | Negative. | |
| Epididymis | Negative. | |
| Female genital | Breast | Negative. |
| Uterus, myometrium | Negative. | |
| Uterus, ectocervix | Negative. | |
| Uterus endocervix | Negative. | |
| Uterus, endometrium | A focal membranous CDH17 staining can rarely occur in individual epithelial cells or groups of epithelial cells. | |
| Fallopian Tube | Negative. | |
| Ovary | Negative. | |
| Placenta early | Negative. | |
| Placenta mature | Negative. | |
| Amnion | Negative. | |
| Chorion | Negative. | |
| Skin | Epidermis | Negative. |
| Sebaceous glands | Negative. | |
| Muscle/connective tissue | Heart muscle | Negative. |
| Skeletal muscle | Negative. | |
| Smooth muscle | Negative. | |
| Vessel walls | Negative. | |
| Fat | Negative. | |
| Stroma | Negative. | |
| Endothelium | Negative. | |
| Bone marrow/ lymphoid tissue | Bone marrow | Negative. |
| Lymph node | Few CDH17 positive inflammatory cells may be seen. | |
| Spleen | Negative. | |
| Thymus | Negative. | |
| Tonsil | CDH17 positive inflammatory cells are regularly seen, especially in the vicinity of squamous epithelium of surface and crypts. | |
| Remarks | A weak to moderate membranous CDH17 staining can be seen in a small subset of inflammatory cells. CDH17 positive inflammatory cells are often closely associated to epithelial cell layers. They may possibly represent a subset of dendritic cells. |
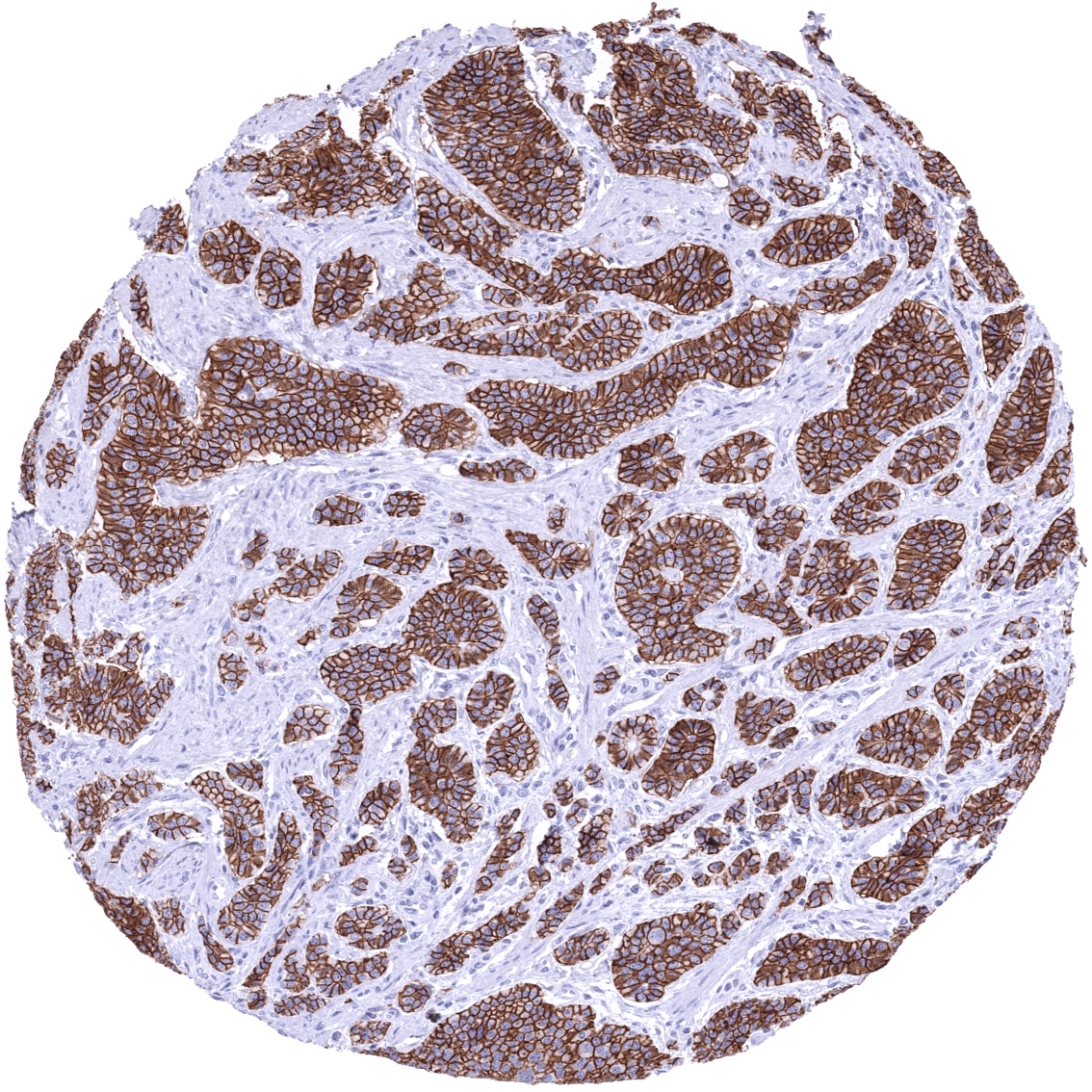
A strong CDH17 staining is seen in all epithelial cells of the small intestine, colorectum and appendix. In these cells, the staining intensity is less intense or even absent at the apical membranes. A moderate CDH17 staining is also seen in the surface epithelial cells of the transitional epithelium of the anal canal. Gallbladder epithelium exhibits a weak to moderate focal membranous staining while intrahepatic bile ducts are CDH17 negative. Few small intrapancreatic excretory ducts show a moderate to strong membranous CDH17 staining. In a few cases a focal membranous staining can be seen in endometrium glands.
The findings described above are this consistent with the RNA data described in the Human Protein Atlas (Tissue expression CDH17)
Positive control = Colon: A strong CDH17 staining should be seen in all epithelial cells.
Negative control = Colon: Non-epithelial cells should not show any CDH17 immunostaining.
Staining Pattern in Relevant Tumor Types
A positive CDH17 immunostaining can be seen in a large fraction of adenocarcinomas derived from the colorectum, stomach, and the esophagus. Several other tumor entities are known to at least occasionally express CDH17. These include adenocarcinoma of the pancreas, mucinous ovarian cancer, cancers of the female genital tract and others.
The TCGA findings on CDH17 RNA expression in different tumor categories have been summarized in the Human Protein Atlas.
Compatibility of Antibodies
No data available at the moment
Protocol Recommendations
IHC users have different preferences on how the stains should look like. Some prefer high staining intensity of the target stain and even accept some background. Others favor absolute specificity and lighter target stains. Factors that invariably lead to more intense staining include higher concentration of the antibody and visualization tools, longer incubation time, higher temperature during incubation, higher temperature and longer duration of the heat induced epitope retrieval (slide pretreatment). The impact of the pH during slide pretreatment has variable effects and depends on the antibody and the target protein.
All images and data shown here and in our image galleries are obtained by the manual protocol described below. Other protocols resulting in equivalent staining are described as well.
Manual protocol
Freshly cut sections should be used (less than 10 days between cutting and staining). Heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7,8 Target Retrieval Solution buffer. Apply MSVA-517M at a dilution of 1:150 at 37°C for 60 minutes. Visualization of bound antibody by the EnVision Kit (Dako, Agilent) according to the manufacturer’s directions.
Agilent / Dako – Omnis
Unmasking IHC EnV FLEX TRS High pH 97°C ; FLEX peroxidase blocking for 3 minutes (32°C), MSVA-517M 1:150 for 30 minutes at 32°C, FLEX+ mouse/rabbit (LINKER) for 10 minutes at 32°C, horseradish peroxidase (HRP) for 30 minutes (32°C), FLEX DAB+Sub-Chromo for 5 minutes (32°C), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Agilent / Dako – Autostainer Link 48
Pretreatment in PT-Link for 30 minutes at 95°C (pH high); FLEX peroxidase blocking for 5 minutes (room temperature), MSVA-517M 1:150 for 20 minutes (room temperature), FLEX+ mouse/rabbit (LINKER) for 15 minutes (room temperature), horseradish peroxidase (HRP) for 20 minutes (room temperature), FLEX DAB+Sub-Chromo for 10 minutes (room temperature), FLEX hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, and a longer incubation time of FLEX+LINKER result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Leica – BOND RX
Dewax at 72°C for 30 seconds; Pretreatment in Bond Epitope Retrieval Solution (ER2 – EDTA pH9) for 20 minutes at 100°C; Peroxidase blocking for 5 minutes (room temperature), MSVA-517M 1:150 for 15 minutes (room temperature), Post primary (rabbit anti mouse) for 8 minutes (room temperature), Polymer (goat anti rabbit) for 8 minutes (room temperature), mixed DAB refine for 10 minutes (room temperature), hematoxylin for 5 minutes (room temperature).
These images reflect stainings by the protocol described above. It is of note that a comparable staining result can also be obtained by different protocols. In general, a longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of Post primary and or the Polymer result in stronger staining, potentially at the cost of more background staining. Modifications of the protocol with a strengthening effect on staining intensity in combination with changes of other parameters that result in lower staining intensity can result in a comparable result as shown above.
Roche – Ventana Discovery ULTRA
Pretreatment for 64 minutes at 100°C (pH 8,4); CM peroxidase blocking for 12 minutes (room temperature), MSVA-517M 1:150 for 20 minutes at 36°C, secondary antibody (anti-mouse HQ) for 12 minutes at 36°C, anti-HQ HRP for 12 minutes at room temperature, DAB at room temperature, hematoxylin II at room temperature for 8 minutes, bluing reagent at room temperature for 4 minutes.
These images depict staining results obtained by the protocol described above. It is of note, that the Ventana machines generally require higher antibody concentrations than other commonly used autostainers because the antibodies are automatically diluted during the procedure. Various other protocols can result in an identical result as shown above. A longer pretreatment, a longer incubation time of the primary antibody, a higher antibody concentration, a higher temperature during incubation, and a longer incubation time of secondary antibody and or the anti-HQ HRP result in stronger staining, potentially at the cost of more background staining.
Potential Research Applications
- The diagnostic utility of CDH17 IHC should be investigated in a large cohort containing as many as possible tumors from as many as possible different entities.
- The clinical significance of CDH17 expression levels in gastrointestinal tumors deserves further investigation.
Evidence for Antibody Specificity in IHC
There are two ways how the specificity of antibodies can be documented for immunohistochemistry on formalin fixed tissues. These are: 1. Comparison with a second independent method for target expression measurement across a large number of different tissue types (orthogonal strategy), and 2. Comparison with one or several independent antibodies for the same target and showing that all positive staining results are also seen with other antibodies for the same target (independent antibody strategy).
Orthogonal validation: For the antibody MSVA-517M specificity is suggested by the strong concordance of the immunostaining data with data from three independent RNA screening studies, including the Human Protein Atlas (HPA) RNA-seq tissue dataset, the FANTOM5 project, and the Genotype-Tissue Expression (GTEx) project, which are all summarized in the Human Protein Atlas (Tissue expression CDH17). RNA expression largely predominated in colorectum, small intestine, duodenum, and the appendix, the organs where a strong CDH17 staining was observed in all epithelial cells by MSVA-517M. An additional, low level RNA expression in smooth muscle tissues could not be confirmed by either MSVA-517M or other CDH17 antibodies. It is possible that smooth muscle samples derived from the gastrointestinal tract were due to a contamination of some of these samples by (CDH17 expressing) epithelial cells. Additional CDH17 stainings observed in gallbladder epithelium and a fraction of endometrial glands was not supported by the available RNA data. These structures constitute small subsets of the total amount of cells in these organs and may have been largely underrepresented and therefore not detected in RNA analyses.
Comparison of antibodies: True expression of CDH17 in gallbladder epithelium and a fraction of endometrial glands is corroborated by comparison with another commercially available independent second antibody (termed “validation antibody”). Comparative images are shown below.